New Janus Particle Additives
for Creating Novel, Water-Resistant and Self-Stratified Coatings
Image courtesy of Iowa State University.
By Shan Jiang, Ph.D., Assistant Professor; Yifan Li, Ph.D. Candidate; and Rebecca Mort, Ph.D. Candidate, Materials Science and Engineering, Iowa State University, Ames, IA
The coating industry has dramatically changed since the 1950s, when organic solvent-based coatings were gradually replaced with waterborne emulsion latex polymer coatings. Waterborne coatings offer great advantages for health and the environment, but still present challenges in terms of coating performance, including water resistance, stability and surface hardness. The major obstacles to overcoming these challenges lie within the contradictions of desired properties before and after drying. For example, it is beneficial for latex particles to be attracted to water (hydrophilic) to maintain a stable dispersion prior to applying a coating to a surface. However, the opposite is desired after a coating has been applied and dried, when water repellence (hydrophobicity) is required. Therefore, a solution that can create a hydrophobic surface after drying while remaining predominantly hydrophilic in dispersion is needed to make waterborne coating technology more versatile.
Another example is the influence of glass transition temperature of the resin (Tg). For improving the film formation during the drying process, low Tg is desired. However, for improving surface hardness after paint is dried, high Tg is required. One potential solution is to stratify a layer of high-Tg binder particles to the surface of the coating layer to boost the hardness, while maintaining the majority of the bulk binder particles with lower Tg for good film formation properties. The recent development of novel Janus particles additives offers promise of addressing these challenges through a unique self-stratification process.
Recent research conducted at Iowa State University has developed an innovative coating additive made from amphiphilic Janus particles that can alter the surface properties of a waterborne coating system.¹ Janus particles are named after the ancient Roman god, having different chemical compositions on each side of a single particle. This means that one side of a particle is hydrophilic while the opposite side is hydrophobic. Figure 1 shows the Janus particle structure. When mixed with hydrophilic binder particles, the Janus particles rapidly migrate to the surface of a coating – a process called “active self-stratification.” Once stratified, the hydrophilic half of the Janus particles will continue to adhere to the binder that makes up the rest of the coating. The hydrophobic half then faces the air interface, creating a perfect monolayer on the surface of the dried coating. As a result, the surface of the coating has different properties (water resistance) than the bulk. Therefore, this new additive offers a cost-effective and scalable solution to directly alter the surface properties of a coating layer without changing the bulk materials. This will help address many of the challenges in the current waterborne coating technology.
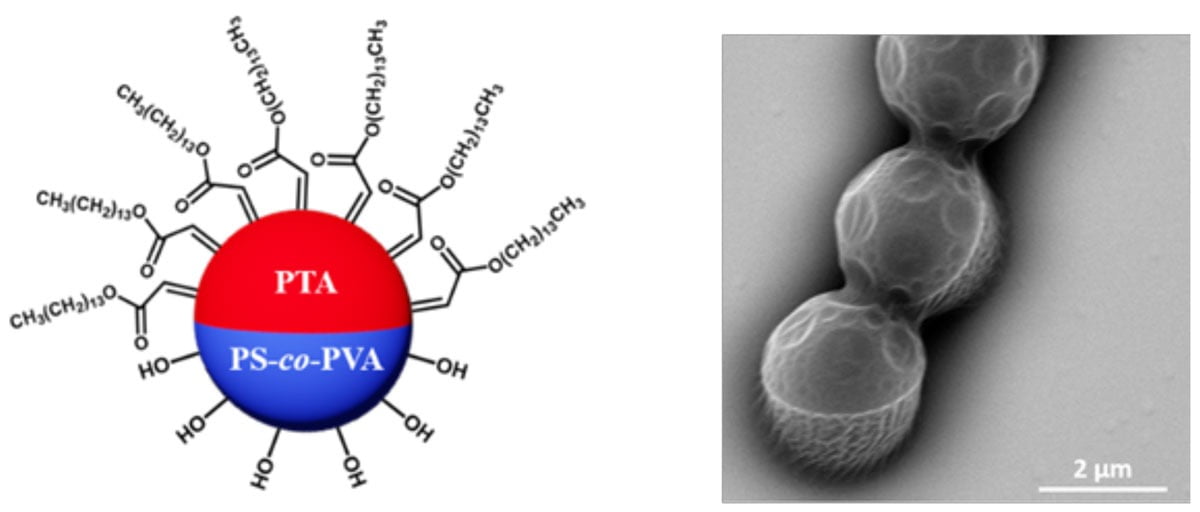
FIGURE 1 ǀ Illustration of Janus particle surface chemistry (left) and scanning electron microscope (SEM) image of Janus particles (right).
Experiment
We discovered the unique “active self-stratification” behaviors of Janus particles and successfully created durable hydrophobic coatings. The novelty of our synthesis lies within the fine control over the Janus particle size and morphology by changing the solvent and surfactant concentration used for seeded emulsion polymerization, enabling us to obtain amphiphilic Janus particles with different sizes. Thus, when mixing Janus particle additives with binder particles or commercial primers, a self-stratifying effect is achieved. Janus particles vigorously accumulate at the air-water interface in fast kinetics, with their hydrophobic sides orienting towards air. Since the hydrophilic side of Janus particles adheres strongly to the binder particles, this new system addresses the weak adhesion issue of conventional hydrophobic coatings. Our method of synthesis is compatible with the economical and scalable emulsion polymerization technique, which has been broadly adopted in the coatings industry.
As shown in Figure 2, with our approach the Janus particles are formulated as a straightforward drop-in additive that can be directly applied to the waterborne latex coating system. This means our method is fully compatible with current commercial products. The advantages are obvious – there is no need to completely redesign the current coating system, and manufacturers can quickly adapt the new method by simply adding Janus particles in their product lines.
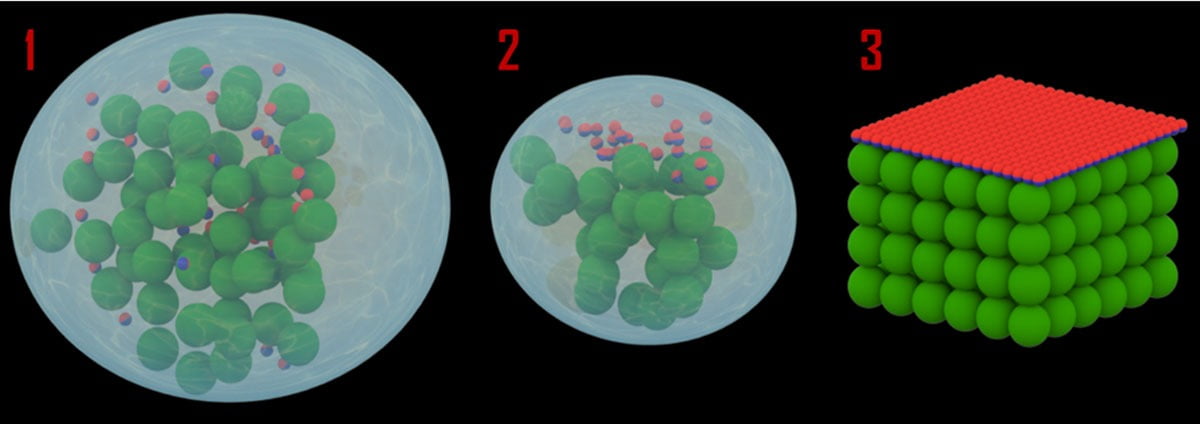
FIGURE 2 ǀ Schematic representation of self-stratified hydrophobic coating formation.
After the mixed coating is fully dried under ambient environment, we can see from Figure 3 that Janus particles exclusively cover the surface and change the surface-related coating properties (Figure 3c). The coating matrix was converted from fully hydrophilic surface (water contact angle ~ 0°), which is made from primer particles to the fully hydrophobic surface (water contact angle ~ 120°). The bulk materials of the coating film remain intact, highlighting the potential advantages of our technology: there is no need to use a typical two-coat system to enhance the coatings properties, which costs additional time, effort and materials.²
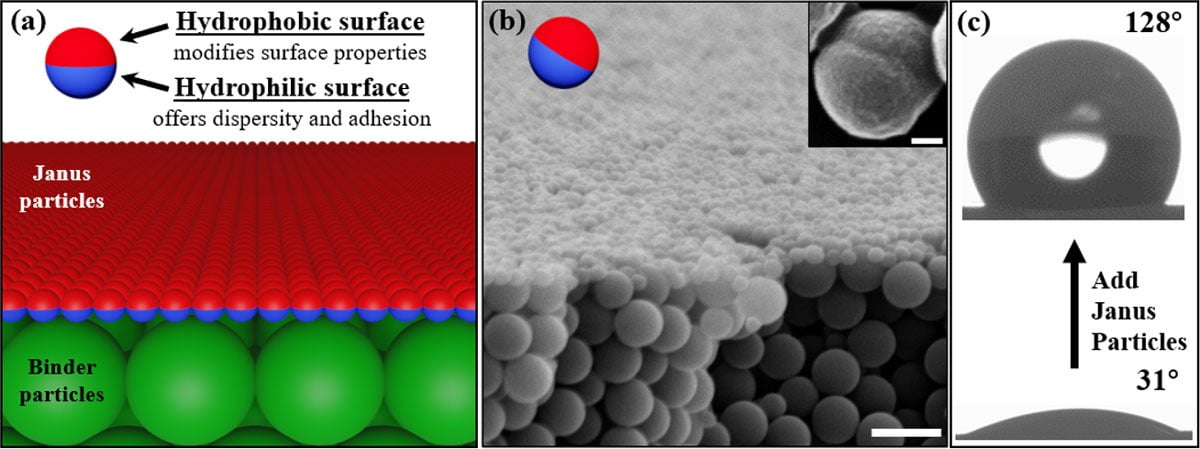
FIGURE 3 ǀ Schematic diagram for the coating structures; b) SEM image of the cross-section view of coating structures. Scale bar is 2 μm. Inset shows the asymmetric morphology of a typical Janus particle. Scalebar is 100 nm; c) Contact angles of the coating surface before and after adding the Janus particles.
Performance Test
The goal of this study is to verify adding Janus particles will not affect the original properties of the coating matrix. Organic solvents (EtOH/THF) were used to rinse the surface of the coating films. Coating films formed by homogeneous hydrophobic particles (with the same surface chemistry as the Janus particles) and binder particles were chosen as a control. Images of the cross-sections of the coating films after rinsing in Figure 4 clearly demonstrate that the self-stratified coating surface maintains its structure integrity, and surface hydrophobicity is unchanged. The resistance highlights the completeness of the densely packed monolayer of amphiphilic Janus particles formed during the self-stratification process and the strong adhesion offered by the phosphate binder particles. In comparison, the control sample that does not possess the stratified structures (Figure 4b) was destroyed in rinsing with solvent (Figure 4d). Since the hydrophobic homogeneous particles randomly dispersed within the binder matrix, they acted as the defects and significantly weakened the coating integrity and adhesion.
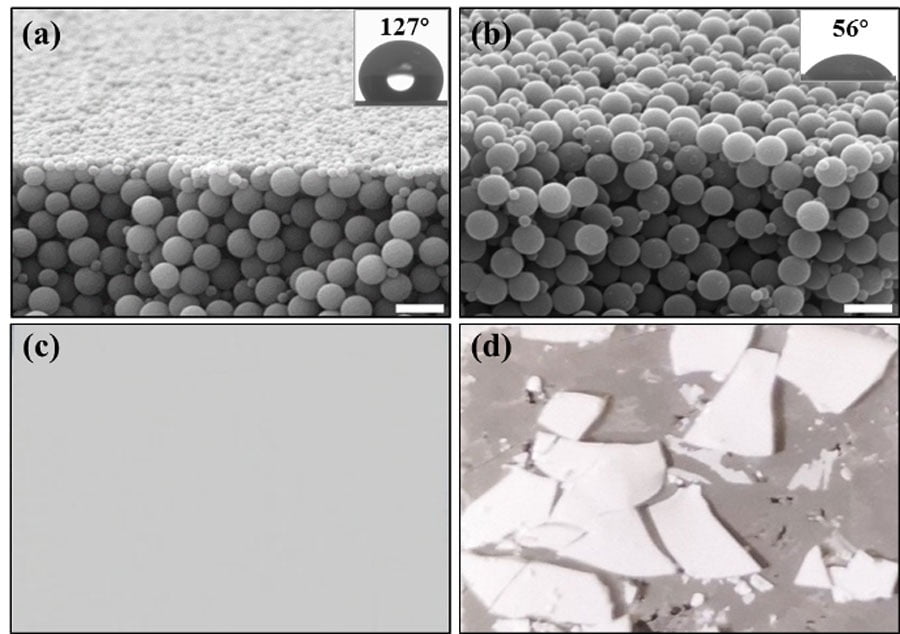
FIGURE 4 ǀ SEM images and photos of the dried coating films after rinsing: a) films formed with Janus particles; b) films with hydrophobic particles; c) Photo of the coating film added with Janus particles after rinse; d) Photo of the coating film added with homogeneous particles after rinse. Scale bars are all 2μm.
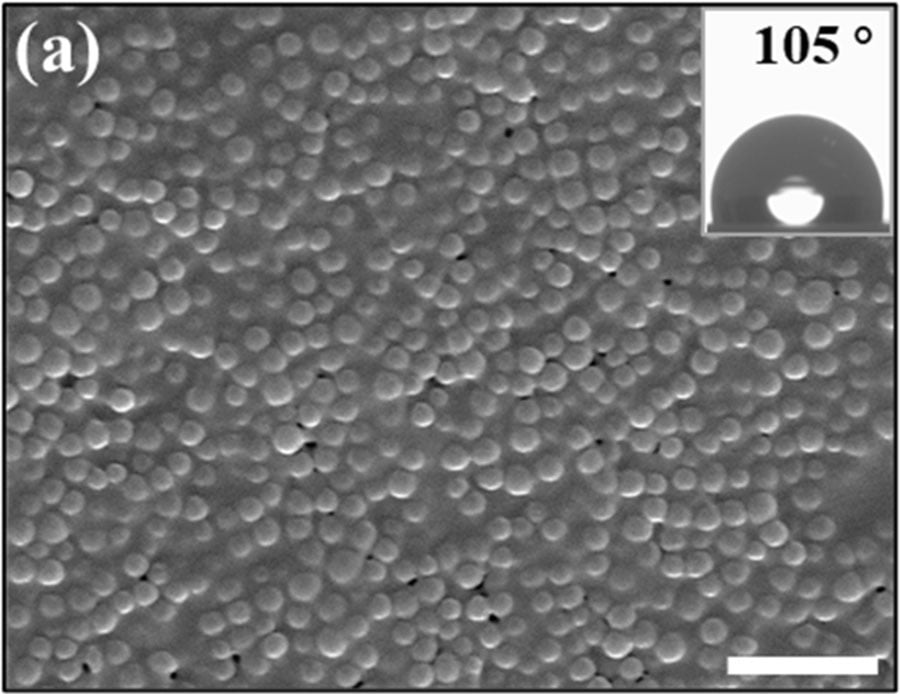
FIGURE 5 ǀ SEM images of coating films formed by commercial primer binder added with Janus particles. Scale bar is 2 μm.
To further test the performance of Janus particles with commercial coating products, Janus particles were applied to a common commercial primer. The successful stratification demonstrates the easy integration of our method into existing products. Janus particles (15% by dry weight) were directly added to the commercial primer. Figure 5 clearly shows self-stratification of Janus particles at the surface of the commercial primer coating. Since the primer binder particles have a glass transition temperature much lower than the room temperature, they form a continuous film after the coating film is dried. Most of the coating surfaces were covered with self-stratified Janus particles, and the contact angle was increased from 0° to 105°. Force characterization also suggests that the surface formed after addition of Janus particles is less tacky, since Janus particle additive has a much higher Tg than bulk binder particles.
Conclusion
In this study, we demonstrate a new coating additive made from Janus particles. These particles can be produced in large quantity with cost-effective emulsion polymerization. Even better, these additive particles can be applied directly to current waterborne coating systems. With a relatively small quantity of Janus particles, they can self-stratify quickly to the surface and create durable hydrophobic coatings with strong adhesion. The approach demonstrated here is economical and commercially scalable. The successful application of Janus particles in commercial coating products showcases the versatility and effectiveness of our approach, and opens a new front for functionalized coatings, which may find broad applications different coating related products.
References
¹ Li, Y.; Liu, F.; Chen, S.; Tsyrenova, A.; Miller, K.; Olson, E.; Mort, R.; Palm, D.; Xiang, C.; Yong, X.; Jiang, S. Self-Stratification of Amphiphilic Janus Particles at Coating Surfaces. Materials Horizons. 2020; 7(8):2047-55.
² Jiang, S.; Van Dyk, A.; Maurice, A.; Bohling, J.; Fasano, D.; Brownell, S. Design Colloidal Particle Morphology and Self-Assembly for Coating Applications. Chemical Society Reviews. 2017; 46(12):3792-807.
