Ready to proof -- Clare 03/14/22
KJ proofed on 3/15 and sent corrections
Revised 3/16
Ready for author
KJ sent corrected article to author on 3/18 - CLEAN
Video: tratong, Creatas Video, via Getty Images
Unsaturated Polyesters as New Solvent-Free Adhesion Promoters for High-Solids Coating Systems
By Ali Javadi, Senior Scientist; and Jim Reader, Senior Technical Manager; Specialty Additives Division, Evonik Corporation, Trexlertown, PA
Polymeric coatings have been extensively used in industrial fields because of their diverse and excellent characteristics. Adhesive properties are probably the most important characteristics of polymeric coatings. These properties are generally categorized into two configurations, namely cohesion and adhesion. When two contacting surfaces are the same, the force required to separate them is identified as cohesion. On the contrary, if two contacting surfaces are different, the separating force is referred to as adhesion. When polymeric coatings are formulated, it is essential to provide for acceptable adhesion features.
Adhesion, as a multi-disciplinary topic, is related to the interatomic and intermolecular interactions present at the interface of two surfaces.1 In polymeric coatings, the adhesion deals with several concepts such as thermodynamics, surface chemistry and physics, polymer chemistry and physics, rheology, and mechanical properties. Understanding the mechanism of adhesion is difficult because of the complexity of the subject.2, 3 In literature, surface morphology, surface wetting, mechanical interlocking, diffusion process, entanglement, acid-base interactions, electrostatic interactions and chemical bonding are among some of the most important parameters studied regarding the mechanisms of adhesion.4
The adhesion properties of polymeric coatings have been studied for decades, and substantial progress has been attained in understanding adhesion mechanisms. In general, the adhesive interactions between the contact surface of a coating and another identical or different surface are determined by the physical and chemical interactions of the macromolecular chains across the interfaces. Different parameters such as the contact time, detachment velocity, temperature and molecular weight (Mw) of polymers have been found to play significant roles in the adhesion properties of organic coatings.
The separation of a coating from a substrate initiates from the occurrence of a microscale crack under applied tensile stress. As the external stress increases, the crack propagates. Ultimately, the development of the crack leads to adhesion failure. In a glassy coating, the crack propagation could be associated with the development of crazing. In such systems, crazing refers to the creation of a network structure with stretched coating strands under applied tensile stress. In an elastomeric coating, it seems the adhesion energy between two surfaces depends on the velocity of crack propagation. In these coatings, the adhesion can be improved by the chemical and physical interactions associated with the interpenetration and entanglement of the polymer chains across the contacting surfaces.5, 6
In glassy polymers, the entanglements of macromolecular chains can typically transfer the endured stress. Theoretical simulations showed that when the degree of polymerization (DP) is less than twice the average number of involved monomers in the entanglements (Ne), the macromolecular chains can be easily pulled out instead of creating a craze. In these situations, the disentanglement does not have a significant contribution to fracture toughness. However, when DP/Ne is greater than 2, a stable craze zone starts to form, indicating the contribution of polymer entanglements to adhesion energy. Because of the macromolecular chain friction, it is also suggested that the dissipated work during the surface fracture and crazing is mostly converted to heat.7
In viscous and viscoelastic polymers, several parameters can affect the polymer chain entanglements across the contacting surfaces and consequently govern the adhesive properties between coatings and substrates. These parameters include the bulk viscoelasticity representing chain mobility, the areal density of polymer chain ends at the interface, and the chemical and physical interactions between polymer chains.6, 8, 9 The mobility of macromolecular chains facilitates the deformation of surfaces and interfaces, resulting in the coalescence of polymer films and the spreading of coatings on the substrates.
In the coating industry, an adhesion promoter is generally used as an additive or a primer to improve the adhesion of coatings to the substrate of interest. An adhesion promoter typically has an affinity for the applied coating and the substrate. This interfacial bridge ideally improves the joint strength and prevents delamination of the coating from the substrate. Without an adhesion promoter, the properties of the applied coatings may not be sufficient to meet the requirements desired for the end products.10 Several theories have been suggested to describe the possible mechanisms by which adhesion promoters can improve adhesions. To date, different adhesion promoters based on organosilanes, organotitanates, zircoaluminates, chlorinated polyolefins, non-chlorinated modified polyolefins, acrylates, aryl/alkyl phosphate esters, ureido-containing monomers, etc. have been reported.11-13 However, the literature is limited regarding the use of amorphous unsaturated polyester (UP) resins as the adhesion promoters in the coatings industry.14
UPs are generally prepared by the condensation of diols with saturated and unsaturated dicarboxylic acids or their corresponding anhydrides. The properties of UPs depend on the ratio and type of the starting raw materials. The polymerizable double bonds used are typically α,β-unsaturated acids, such as fumaric acid, maleic acid and maleic anhydride. The UPs containing the higher contents of the double bonds are more chemically reactive. These UPs polymerize rapidly with intense heat development to give highly crosslinked and comparatively brittle products. To address this issue, the reactive double bonds are diluted by the condensation of saturated aliphatic or aromatic dicarboxylic acids. The prepared UP types differ not only in the components used but also in other parameters including the DP, the ratio of saturated to unsaturated acids, the acid and/or hydroxyl values, and the crosslinking density.
Polyester-based adhesion promoters (PEAPs) are a series of modified UP resins that can partially replace the main binders in coatings formulations to improve their adhesion to difficult substrates. As shown in Figure 1, these UP resins have multiple functional groups that can interact with different substrates, while their main polymeric chains are compatible with the main binders of different coatings to ensure good performance in a wide range of applications. PEAPs can also improve the corrosion resistance of the coatings by slowing or preventing the adhesion failures that lead to film delamination as corrosion develops.
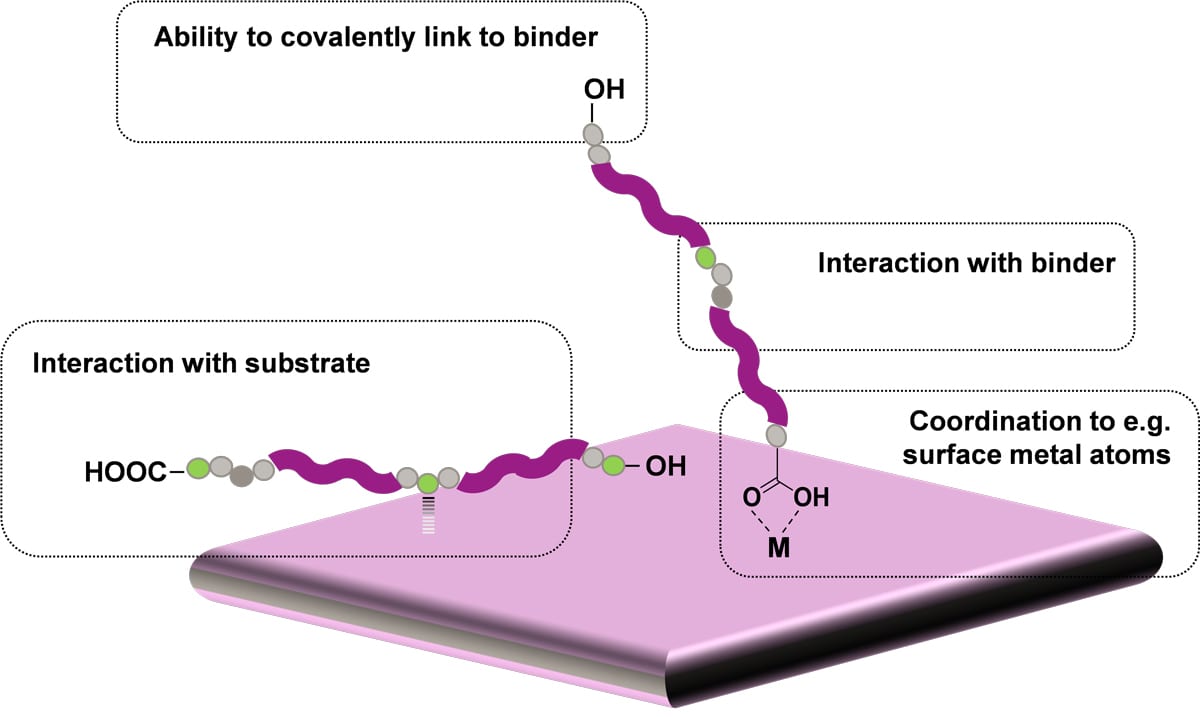
FIGURE 1 ǀ Schematic representation of the possible adhesion mechanisms in the polyester-based adhesion promoters (PEAPs).
Regardless of having good adhesion-promoting characteristics, the polyester-based adhesion promoters relatively have high viscosities. Therefore, these products require dilutions with appropriate solvents to achieve workable viscosities. However, increasingly the stringent environmental regulations limit the amount of volatile organic compounds (VOCs), such as common solvents that can be used in the coatings industry. These regulations limit a formulator’s freedom to use the polyester-based adhesion promoters, as the low viscosities and low solvent contents are mutually exclusive in these systems. To address this issue, Evonik has developed two solvent-free liquid polyester-based adhesion promoters, PEAP 1600 and PEAP 1611, for improving the cohesion and adhesion properties of different coatings. These adhesion promoters provide the coatings with reduced VOC values, good flowabilities and excellent adhesion to different substrates in solvent-free, high solids and radiation-curable formulations. The chemical and physical properties of PEAP 1600 and PEAP 1611 are shown in Table 1.
TABLE 1 ǀ The chemical and physical properties of PEAP 1600 and PEAP 1611.
In this study, the performance of these adhesion promoters, PEAP 1600 and PEAP 1611, was investigated in four different high solids systems, including a 1K alkyd metal topcoat, a 1K melamine-cured polyester stoving enamel, a 2K white polyurethane (PU) topcoat, and a 2K epoxy primer. The properties of these coatings, such as the rheological behavior, gloss, color, adhesion, impact flexibility and corrosion resistance, were investigated in detail.
Experimental
All chemicals and reagents were used as received without further purification. The rheological properties of the prepared samples were measured using an Anton Paar MCR rheometer. The prepared samples were applied by drawdown to have a dry film thickness (DFT) of ~75 µm (~3 mils). Since most properties of coatings are thickness-dependent, it was important to determine the optimum thickness for each application. The methods developed by the American Society for Testing and Materials (ASTM) were used to evaluate the coatings. Following the procedure given in ASTM D3359, the cross-hatch tape test was used to examine the adhesion strength of the coatings to the substrates. The adhesion tests and evaluations were performed after the coatings were cured for seven days. The impact flexibility of the prepared coatings was also evaluated after the curing for seven days, using an Impact Flexibility Tester according to ASTM D2794. The coatings were placed in a salt spray chamber (ASTM B117) for 96 hrs to evaluate their corrosion resistance according to ASTM D1654. The gloss values were measured at 20° according to ASTM D523 by a conventional portable gloss meter BYK micro-TRI-gloss. BYK-mac i Multiangle Spectrophotometer was used to measure the Delta E values of the coatings according to ASTM D2244.
Results and Discussion
Obtaining a suitable application viscosity while simultaneously meeting limitations regarding VOC content is a continuous challenge when developing environmentally friendly high solid coatings. In this study, the adhesion promoters PEAP 1600 and PEAP 1611 reduced the viscosity of liquid samples without any adverse effects on their leveling, sagging control and application properties. This effect was more visibly evident as the solids content of the formulation increased. Figure 2 shows how the new adhesion promoters lower the viscosity values of a 2K polyurethane topcoat with 5% replacement of main binder. Similar trends were seen in the other formulations, such as 1K alkyd metal topcoat, 1K melamine-cured polyester stoving enamel and 2K epoxy primer.
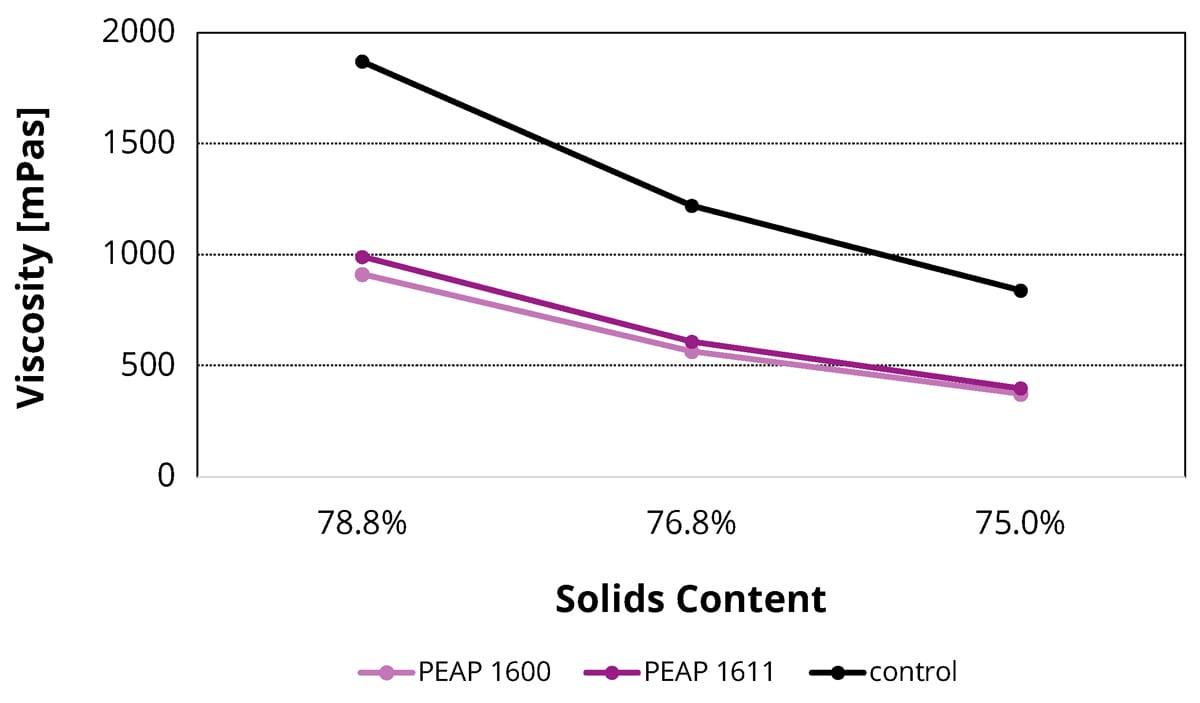
FIGURE 2 ǀ Shear viscosity of the prepared 2K polyurethane topcoat samples with 5% replacement of the main binder with PEAP 1600 and PEAP 1611. The prepared samples have the solids content of 75.0%, 76.8% and 78.8%.
The durability of the prepared coatings was tested by cross-hatch tape adhesion test (ASTM D3359). This test is generally used to examine the adhesion strength of the coatings to the substrates. The cross-hatch tape adhesion test results in 1K alkyd metal topcoat formulation are shown in Table 2. As shown, significant improvements in adhesions on different substrates were achieved by replacing 3, 5 or 10 wt.% of the main binder with the PEAP 1600 and PEAP 1611. Similar results were also seen in the other formulations, such as 1K melamine-cured polyester stoving enamel, 2K white PU topcoat and 2K epoxy primer.
TABLE 2 ǀ The cross-hatch tape adhesion test results in a 1K alkyd metal topcoat formulation.
ASTM D2794 is a useful test in predicting a coating's resistance to an external impact. It provides a method for rapid deformation of a coating as well as subsequent evaluation of deformation effects. After the application and curing of the coatings, a standard weight is dropped a distance to strike an indenter that deforms the cured coating and its substrate. The point at which the failure occurs can be determined by gradually increasing the distance the weight drops, usually 2.5 cm (1.0 inch) at a time. In general, coatings fail by cracking, which is made more visible using a magnifier or a tape-pull test to evaluate the amount of coating removed. Once a visible crack has been identified, the test is usually repeated five times at that level, along with five times below and above that level. It is important to conduct these confirmation tests in a random order. Table 3 shows the impact flexibility results of the prepared 2K white PU coatings, using a 1.0 Kg standard weight dropping from the certain distances of 0.2 and 0.5 m. As shown, the 2K PU coatings containing PEAP 1600 and PEAP 1611 showed better deformability and impact resistance compared to those without these adhesion promoters. Such an improved elasticity was also noticed in the other formulations such as 1K alkyd metal topcoat, 1K melamine-cured polyester stoving enamel and 2K epoxy primer.
TABLE 3 ǀ The impact test results in a 2K white PU coating formulation.
The salt spray test is a standardized accelerated corrosion test used to evaluate the corrosion resistance of coatings. In this method, the substrate to be tested is usually metallic and finished with a coating that is intended to provide some degree of corrosion protection to the underlying substrate. After a pre-determined period, the appearance of the corrosion products, such as iron oxide or other metal oxides, is evaluated. The salt spray test duration depends on the corrosion resistance of the coatings. In general, more corrosion-resistant coatings need longer periods of testing before the appearance of corrosion. In this study, the prepared coatings were placed in a salt spray chamber for 96 hrs to evaluate their corrosion resistance per ASTM D1654. The tested coatings were a 2K PU topcoat (blank), a 2K PU topcoat containing 5 wt.% of PEAP 1600, and a 2K PU topcoat containing 5 wt.% of PEAP 1611. As shown in Figure 3, in comparison with the blank, both the PEAP 1600 and PEAP 1611 minimized the corrosion by showing 38% and 25% less delamination areas, respectively. In the same way, these adhesion promoters could delay the steel panel corrosion in the other formulations.
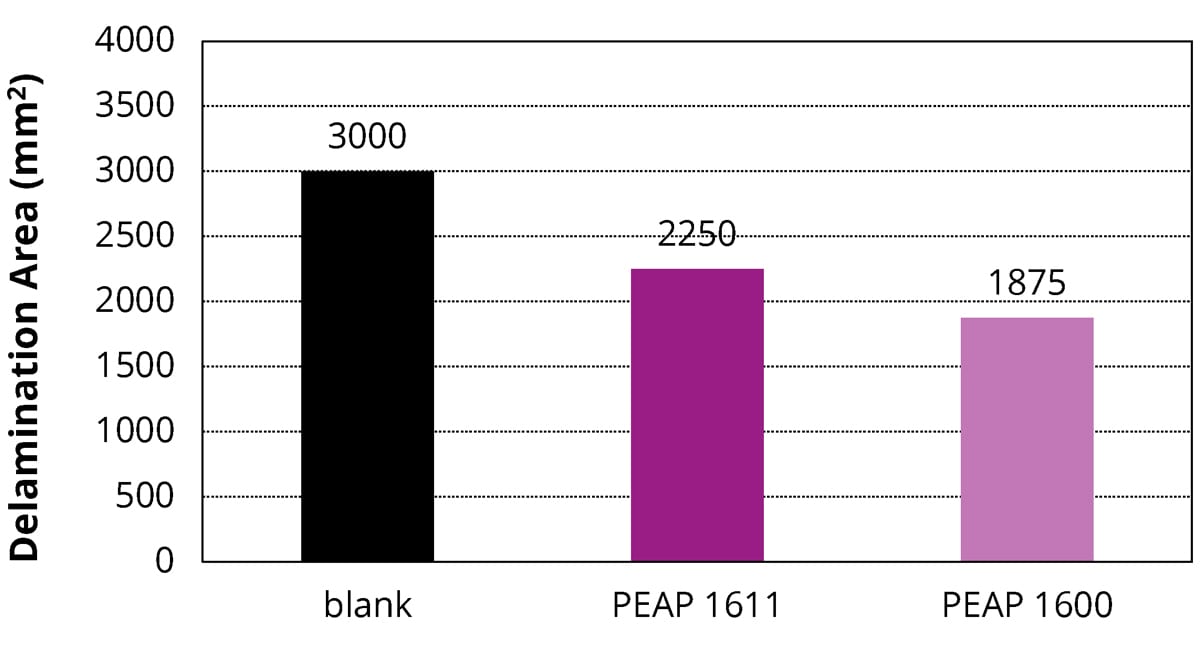
FIGURE 3 ǀ Delamination area (mm2) of the prepared coatings on steel substrates subjected to the salt spray test for 96 hrs. Compared to that of blank (2K PU topcoat formulation), the use of adhesion promoters PEAP 1600 and 1611 minimized the corrosion by 38% and 25% less delamination areas, respectively.
UV light is generally responsible for most of the photodegradation of coating materials exposed outdoors. The fluorescent lamps of UV testers simulate the critical UV lights and realistically reproduce the physical damage caused by sunlight. Some of the most important damage includes loss of gloss, color change, hazing, cracking, chalking, crazing, blistering and strength loss. In this study, special fluorescent UV lamps in the UVA portions of the spectrum were used to evaluate the prepared coatings. The 20° gloss values of the coatings were measured at 250, 500, 750 and 1,000 hrs of the exposure time. Figure 4 shows the results in a 2K PU topcoat formulation without stabilizer (a) and with Tinuvin® 1130 stabilizer (b). As shown, adding the adhesion promoters PEAP 1600 and PEAP 1611 did not have significant effect on the light stability of the coatings.
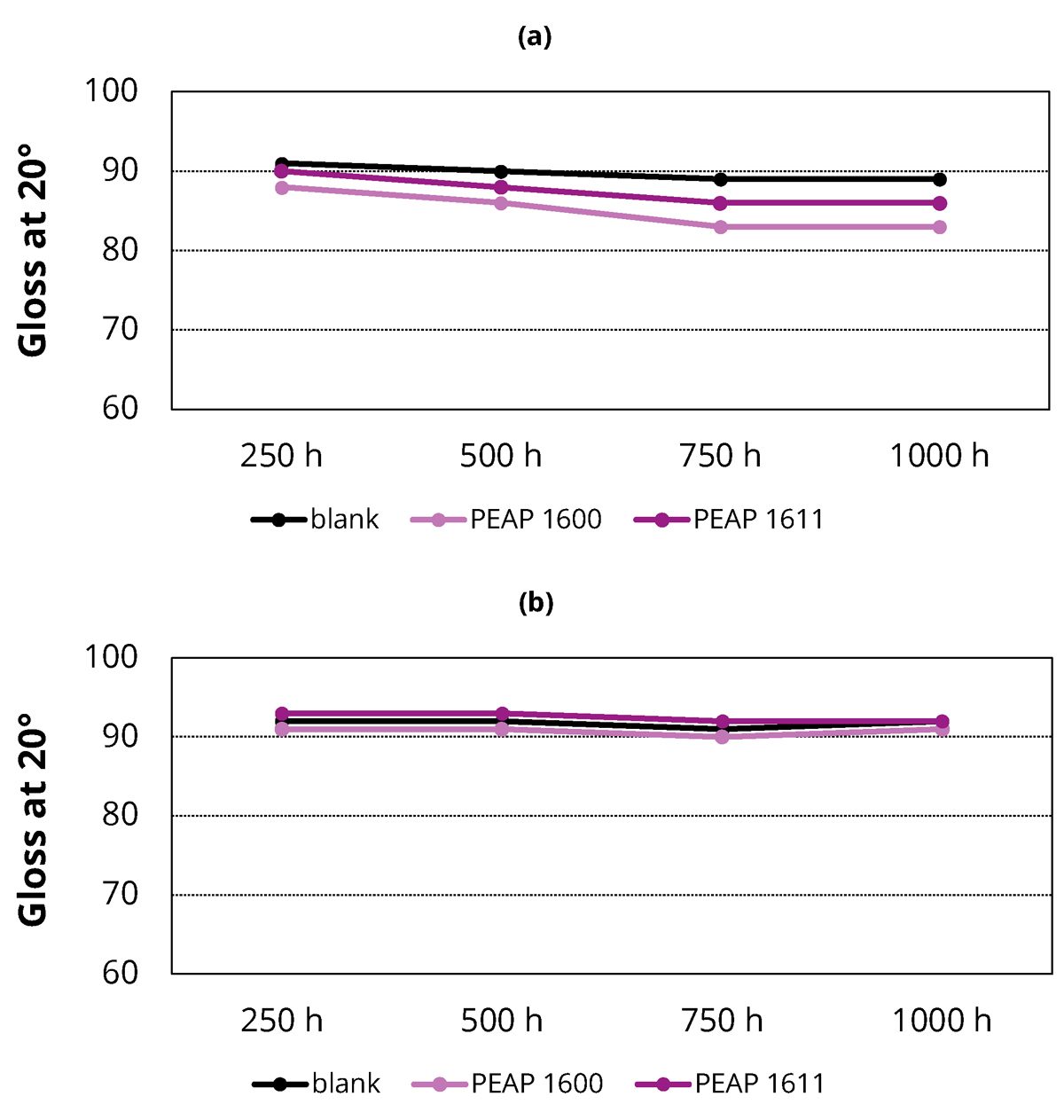
FIGURE 4 ǀ 20° gloss values of the prepared coatings without stabilizer (a) and with Tinuvin 1130 stabilizer (b) after being exposed to the UVA light for 250, 500, 750, and 1,000 hrs. PEAP 1600 and PEAP 1611 did not have significant effect on the light stability of the tested coatings.
Conclusions
Adhesion promoters PEAP 1600 and PEAP 1611 are based on modified unsaturated polyester resins. The performance of these adhesion promoters was studied in different high solids systems, including a 1K alkyd metal topcoat, a 1K melamine-cured polyester stoving enamel, a 2K white polyurethane (PU) topcoat and a 2K epoxy primer. In these coatings, the main binders were partially replaced with PEAP 1600 or PEAP 1611. These adhesion promoters reduced the viscosity of liquid samples without adverse effects on their rheological properties. Significant improvements in adhesion on different substrates were achieved by partial replacing of the main binders with these adhesion promoters. Also, the prepared coatings containing PEAP 1600 or PEAP 1611 showed light stability, and better impact and corrosion resistance compared to those without these adhesion promoters.
References
1 Poisson, C., et al. Optimization of PE/binder/PA extrusion blow-molded films. II. Adhesion properties improvement using binder/EVA blends. 2006. 101(1): p. 118-127.
2 van der Leeden, M.C.; Frens, G. Surface Properties of Plastic Materials in Relation to Their Adhering Performance. 2002. 4(5): p. 280-289.
3 Awaja, F., et al. Adhesion of Polymers. Progress in Polymer Science, 2009. 34(9): p. 948-968.
4 Wypych, G., 2 - Mechanisms of Adhesion, in Handbook of Adhesion Promoters, G. Wypych, Editor. 2018, ChemTec Publishing. p. 5-44.
5 Ducrot, E., et al. Toughening Elastomers with Sacrificial Bonds and Watching Them Break. Science, 2014. 344(6180): p. 186-9.
6 Gong, L., et al. Fundamentals and Advances in the Adhesion of Polymer Surfaces and Thin Films. Langmuir, 2019. 35(48): p. 15914-15936.
7 Wool, R.P. Polymer Entanglements. Macromolecules, 1993. 26(7): p. 1564-1569.
8 Zeng, H., et al. Adhesion and Friction of Polystyrene Surfaces Around Tg. Macromolecules, 2006. 39(6): p. 2350-2363.
9 Klein, J. The Interdiffision of Polymers. Science, 1990. 250(4981): p. 640-6.
10 Wypych, G., 1 - Introduction, in Handbook of Adhesion Promoters, G. Wypych, Editor. 2018, ChemTec Publishing. p. 1-3.
11 Wypych, G., 7 - Properties of Adhesion Promoters, in Handbook of Adhesion Promoters, G. Wypych, Editor. 2018, ChemTec Publishing. p. 101-138.
12 Wypych, G., 8 - Selection of Adhesion Promoters for Different Substrates, in Handbook of Adhesion Promoters, G. Wypych, Editor. 2018, ChemTec Publishing. p. 139-175.
13 Wypych, G., 9 - Selection of Adhesion Promoters for Different Products, in Handbook of Adhesion Promoters, G. Wypych, Editor. 2018, ChemTec Publishing. p. 177-210.
14 Gloeckner, P., et al. Adhesion Promoter Additive Comprising an Unsaturated, Amorphous Polyester, in United States Patent, USPTO, Editor. 2004, Evonik Operations GmbH.