Niki - the Up Next photo and headline need to be updated. Thanks. revised 03/26-nb
A High-Efficiency, Metal-Free Catalyst
for Silane-Modified Polyurethane Applications
Photo credit: runna10, iSock, via Getty Images
By Jingguo Shen, Technical Manager, Advanced PU Materials Group, Evonik Corporation, Trexlertown, PA
Silane-modified polyurethanes (SPUR) systems find increasingly extensive utilizations in adhesive, sealant, and coating products.1-5 These hybrid polymers take advantage of the synergy between silane and urethane chemistries and, therefore, enjoy a plethora of desirable process and end-use properties, as highlighted in Figure 1. Such properties include: moisture-curable and compatible with 1K (one-component) formulations, which are easy to use and DIY-friendly; enhanced polyurethane-level mechanical properties such as tear resistance; isocyanate-free application with minimum EH&S concerns; bubble-free curing process with less sensitivity to humidity impact and system defects, even in thick layers; excellent adhesion properties with silane moieties serving as internal primer; and printable-cured SPUR surface.
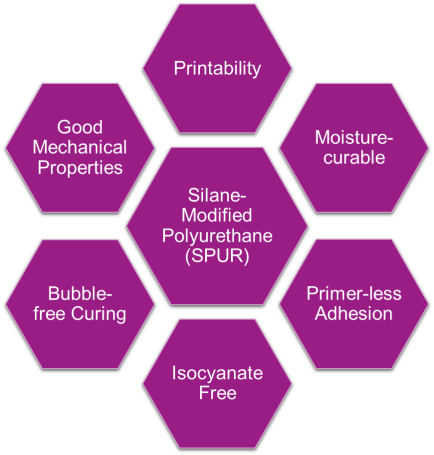
FIGURE 1 ǀ Advantages of SPUR hybrid systems based on silane and urethane chemistries.
Chemically, SPUR systems rely on the hydrolysis and condensation of alkoxysilyl moieties to build up polymer molecular weight and/or crosslink density and, correspondingly, the mechanical properties. More specifically, SPUR cures in multiple steps, as outlined in Figure 2. First is the hydrolysis of the alkoxysilanes, generating silanol groups and alcohols; and second, condensation among silanol groups, as well as silanol and alkoxysilyl groups, creating siloxane bonds, with water and alcohols as the side products.6,7 Overall, though the reaction with water (hydrolysis) is a prerequisite to the ensuing curing steps, both hydrolysis and condensation reactions are believed to occur concurrently throughout the cure process. The important parameters that affect the cure efficiency are the specific chemical nature and relative content of the silyl moieties, the availability of water in ambient environment (relative humidity), system pH, temperature, and catalyst type.
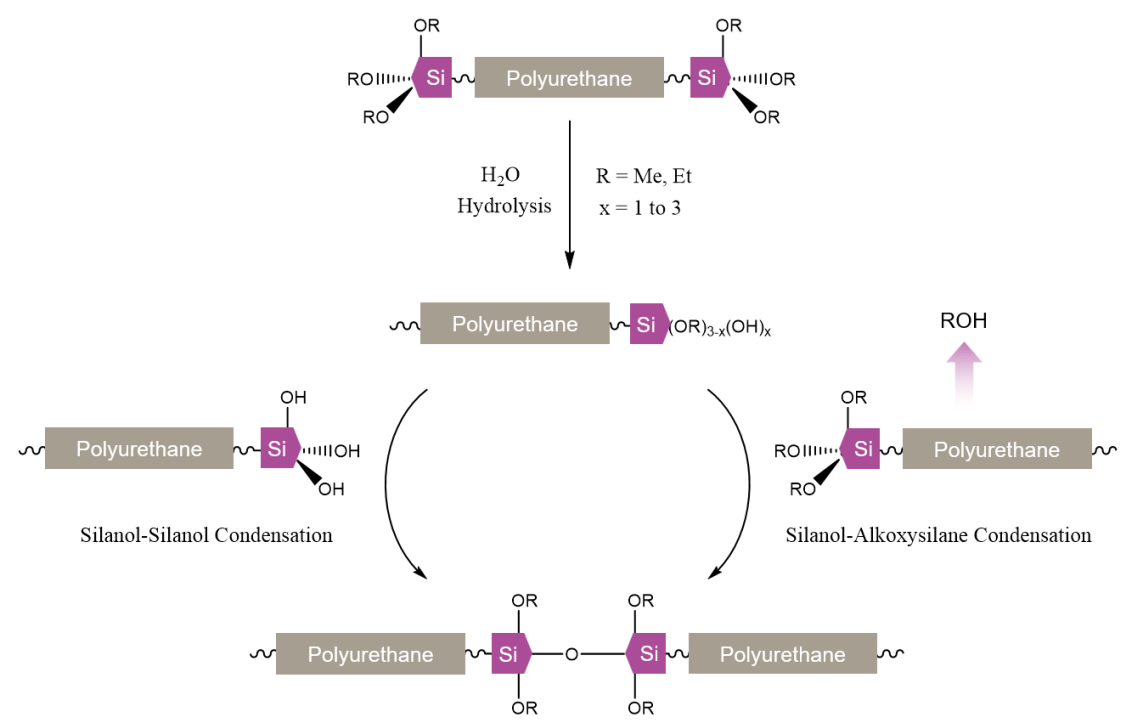
FIGURE 2 ǀ Hydrolysis and condensation reactions in SPUR systems.
It is well understood that virtually all Brønsted acids and bases, as proton donors and acceptors respectively, can accelerate the silane hydrolysis and condensation reactions. However, many of them are typically avoided in SPUR applications, due to various undesirable side effects, such as reactions with treated fillers, discoloration via oxidation, unpleasant odor, or poor compatibility with SPUR network, which in severe cases can lead to migration or exudation of the catalyst onto the cured polymer surface. Another group of catalysts based on organometallics with better catalytic efficiency and compatibility are widely employed in SPUR applications. Among them, organotin compounds such as dibutyltin dilaurate (DBTDL) and dioctyltin dilaurate (DOTDL) are in general considered the industry standard, and best catalyst references for SPUR systems.8 However, most of these organotin catalysts are of high EH&S concern, due to their unfavorable toxicological profiles. Regulatory agencies have placed increasingly restrictive limitations on the use of dibutyltin (DBT) and dioctyltin (DOT) compounds in formulated products. For example, E.U. REACH regulation limits DBT and DOT usage in most applications to less than 0.1% based on tin weight. In anticipation of further restrictions, many SPUR formulators are proactively opting for tin-free catalysis solutions. Additionally, most organotin compounds are in general ineffective in SPUR systems functionalized with ethoxysilyl groups, which are significantly less reactive than their methoxysilyl counterparts.
Herein, Evonik reports a novel, tin-free catalyst, Catalyst V36, which delivers up to an order-of-magnitude-higher reactivity in SPUR applications than conventional catalysts. Performance results on its catalytic activity, formulation and storage stability, UV/light stability, and impact on SPUR system end-use properties are discussed.
Experimental
All chemicals were supplied by Evonik Corporation unless otherwise noted. SPUR formulations were prepared by mixing with a Flak-Tek SpeedMixer. SPUR skin-formation time refers to the time period that elapses following the application of the SPUR formulation until the polymer surface has cured to the extent at which touching the surface of the curing mixture with a gloved finger no longer causes material being transferred onto the glove, and was collected under controlled temperature and residual humidity conditions in a Lunaire steady-state stability chamber CEO-916-4. The 24-hour cure-depth measurement was conducted by casting the SPUR formulation into a custom-made Teflon trench with depth continuously increasing from 0 to 10 mm, and checking the dry-through depth following a 24-hour cure period in the Lunaire chamber. The SPUR films were prepared by casting in a Teflon mold followed by curing in the Lunaire chamber. Tensile data was collected on an Instron 5900R universal testing system following ASTM D638. Viscosity was measured at 25 °C by a Brookfield viscometer DV2T fitted with a Brookfield AMETEK thermosel for temperature control. UV exposure of test SPUR films was carried out using a QUV accelerated weathering tester equipped with a UVA (340 nm) lamp. Color of SPUR films was measured with a BYK-Gardner color meter. Tinuvin 292 was utilized as HALS (hindered amine light stabilizer) in some test formulations.
Results and Discussion
For real-world 1K-SPUR applications, the analysis and characterization of the efficacy of a SPUR catalyst is complicated, as the cure process is typically under both kinetic control, where the silane hydrolysis and condensation reactions occur simultaneously and compete with each other among other side reactions, as well as diffusion control, due to the inherently complex cure behavior where moisture permeation is involved. More specifically, the kinetically more-active catalysts in general are expected to form a skin layer faster than their less-active counterparts, and consequently the quicker vitrification of the superficial layers hinders the further diffusion of moisture as well as the release of alcohol side products, thus may potentially slow down the overall cure-through process.
The high-efficiency tin-free catalyst, Catalyst V36, was tested in representative moisture-cure 1K-SPUR formulated systems, in comparison to DBTDL as the reference catalyst. Moisture-cure rates in controlled (temperature and relative humidity) ambient conditions were characterized with skin-formation time, as well as cure-depth measurement, with the former being a substantially kinetic metric, while the latter reflecting fundamentally more diffusion-control effects.
Catalytic Efficiency in SPUR Based on Trimethoxysilanes
A model SPUR system based on trimethoxylsilanes was prepared according to the formulation listed in Table 1. Polymer ST 80 is a high-modulus SPUR polymer based on polypropylene oxide polyurethane backbone terminally functionalized with trimethoxysilane groups. Catalysts and their use levels were varied accordingly.
TABLE 1 ǀ SPUR formulation based on trimethoxysilanes.
The average measured cure-activity data is plotted in Figure 3. At the equal catalyst use levels of both 0.1% and 0.3%, the SPUR system catalyzed with Catalyst V36 reached surface dryness considerably faster than DBTDL, with an average skin-formation time about 20-30% of that of the corresponding DBTDL systems respectively, indicating its significantly higher catalytic efficiency, in terms of the hydrolysis/condensation-reaction kinetics. With regard to the cure-through data, it is remarkable to note that despite the much faster skin formation observed in the Catalyst V36 SPUR system, it also had 24-hour cure depth comparable to the DBTDL controls, suggesting that the tested model systems were likely not under dominant diffusion control.
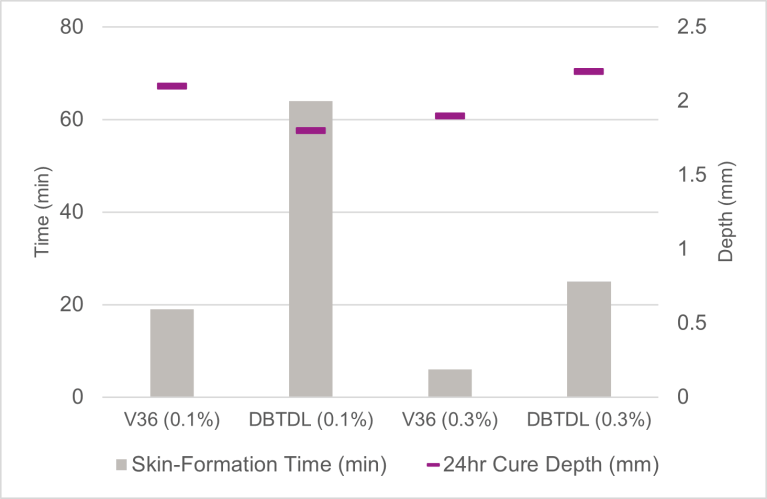
FIGURE 3 ǀ Skin-formation time and 24-hour cure depth of model trimethoxysilane SPUR system catalyzed with Catalyst V36 and DBTDL (at 0.1% and 0.3%, 25 °C / 50% RH).
Mechanical properties of the cured model SPUR systems as indicated by representative tensile test data, as shown in Figure 4, appeared to be comparable for both catalysts. At adjusted catalyst-use levels where both systems had a similar skin-formation time, Catalyst-V36-catalyzed samples, on average, showed slightly higher tensile at break and Young’s modulus values, while lagged slightly behind the DBTDL sample in elongation at break. The minute difference in tensile properties suggests that Catalyst V36 may facilitate a slightly higher degree of crosslinking in the model SPUR system.
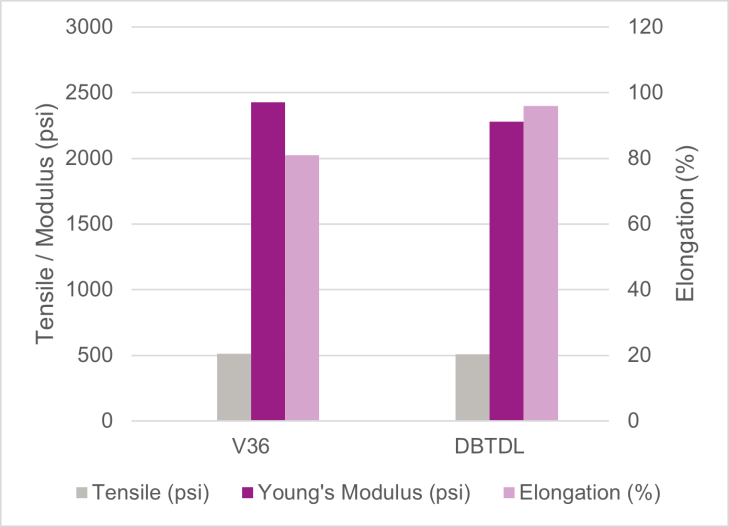
FIGURE 4 ǀ Tensile test data for model trimethoxysilane SPUR system catalyzed with Catalyst V36, DBTDL at 25 °C / 50% RH.
In summary, in terms of raw catalyzed reaction rates of silane hydrolysis and condensation reactions, the novel tin-free Catalyst V36 outperforms the organotin reference catalyst DBTDL by a sizeable margin in the model trimethoxylsilane-based SPUR formulations. It is also remarkable that the heightened catalytic efficiency appears to have no negative impact on the cure-through rates, as well as the general cured physical properties. Overall, Catalyst V36 is well positioned to replace tin catalysts in trimethoxysilane SPUR applications.
Catalyic Efficiency in SPUR Based on Ethoxysilanes
Although at present methoxysilane-based polymers account for the vast majority of the commercial SPUR applications, their ethoxysilane counterparts are of research and development interest as a more EH&S-friendly alternative. As indicated in the reaction steps of the silane hydrolysis and condensation reactions shown in Figure 2, when the R groups are methyl, one of the side products is methanol, a harmful volatile compound that is subject to regulations in many jurisdictions worldwide. Ethoxysilane-based SPUR polymers represent the most-effective approach to fundamentally eliminate the methanol emission issue, as they generate ethanol as the relatively safer by-product. However, very importantly in the context of SPUR catalysis, the organotin catalysts, in general, are not capable of accelerating the ethoxysilane systems at reasonable use levels, which at least partially contributes to their currently slow adoption in the industry. Therefore, the catalytic efficiency of the tin-free Catalyst V36 was evaluated in a SPUR formulation based on ethoxysilane-functionalized polymers, along with the DBTDL controls.
The model formulation is listed in Table 2. Tegopac bond 160 is a medium-modulus SPUR polymer based on polypropylene-oxide polyurethane backbone laterally functionalized with triethoxysilane groups.
TABLE 2 ǀ SPUR formulation based on triethoxysilanes.
The test data characterizing cure activities of the model ethoxysilane SPUR systems is plotted in Figure 5. Based on the skin-formation times, the advantage of Catalyst V36 in catalytic efficiency over DBTDL is apparent, reaching the comparable level of surface dryness in significantly less amount of time, and at much lower use levels. In line with reports in the literature, DBTDL, similar to other organotin-based catalysts, while in general provides sufficient cure acceleration in methoxysilane systems, appears to be essentially ineffective in ethoxysilane formulations. In the tested model system based on triethoxysilane polymers, at both 1% and 3% levels, DBTDL failed to achieve skin-formation in less than 10 hours, while even at 1% the tin content is already almost twice the maximum allowed by REACH regulation. The cure-through rates trended correspondingly, with excellent cure depth observed for the Catalyst V36 systems versus the DBTDL controls, which remain mostly uncured over a 24-hour period. Compared to the methoxysilane system results, the advantage of Catalyst V36 in raw-catalytic efficiency over tin catalysts is even more pronounced in the ethoxysilane applications.
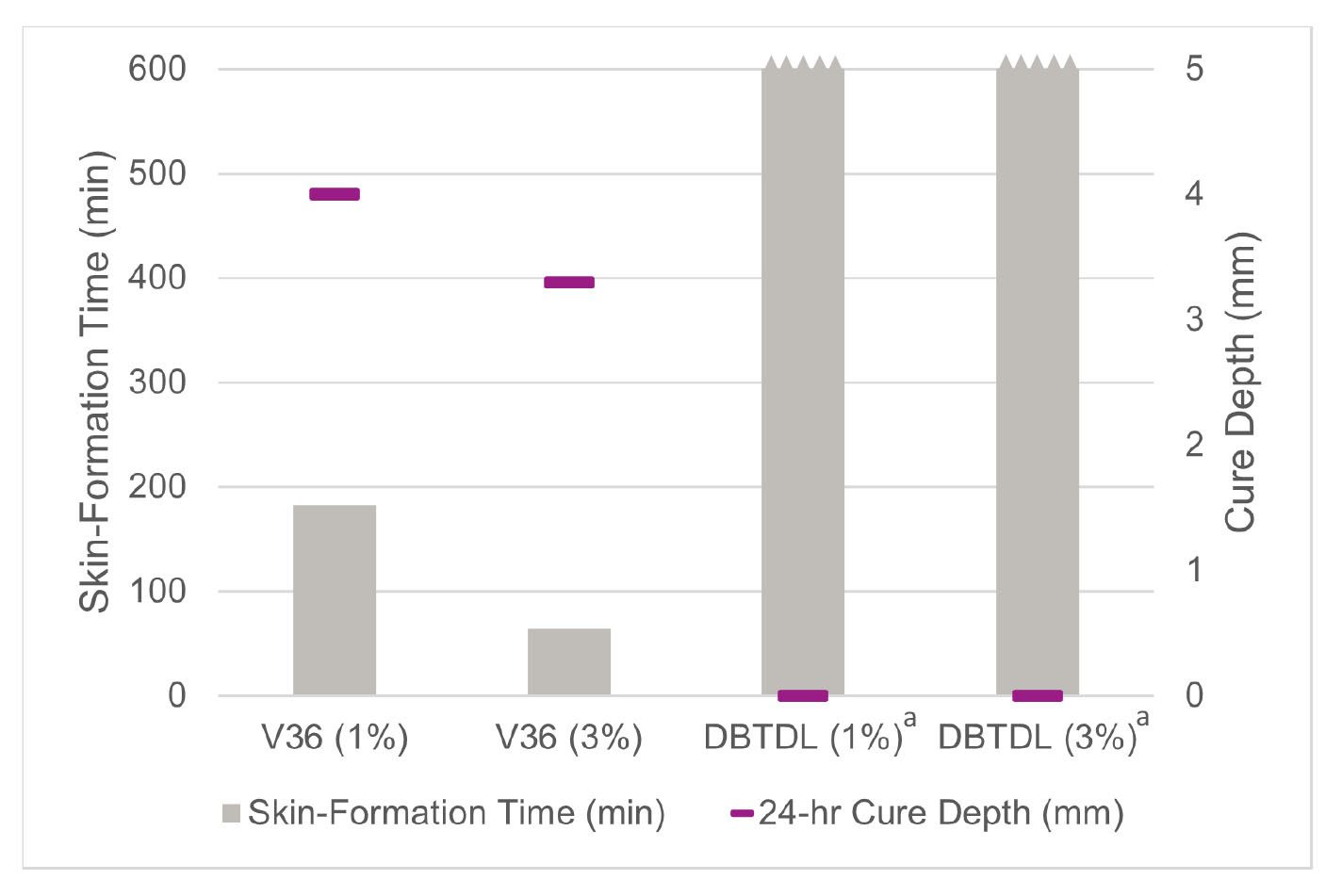
FIGURE 5 ǀ Skin-formation time and 24-hour cure depth of model triethoxysilane SPUR system catalyzed with Catalyst V36 and DBTDL at 25 °C / 50% RH (aDBTDL systems not cured in 10 hours).
Furthermore, the lack of efficacy of the industry-standard organotin catalysts likely represents one of the main barriers for the market to further adopt the more EH&S-friendly ethoxysilane SPUR systems. In other words, a tin-free SPUR catalyst that demonstrates high efficiency in ethoxysilane systems, such as Catalyst V36, may be one of the key enablers of the ethoxysilane technology, thereby mitigating simultaneously the methanol- and organotin-related regulatory concerns.
Catalyst Impact on System Stability
For a 1K SPUR system, a successful catalyst needs to efficiently accelerate the curing process, but perhaps more importantly, do so with minimum and ideally no negative impact on the overall stability of the formulated product. The main performance metrics of such catalyst stability include: drift of catalytic efficiency and change of phase consistency; viscosity or color in the formulated system in storage over time; as well as the degree of catalyst migration to the surface and color change in the cured SPUR parts.
The reactivity drift of a model trimethoxysilane SPUR formulation catalyzed by Catalyst V36 or DBTDL was characterized with its skin-formation time as well as cure-through rate, as measured over a 4-week RT storage period (Figure 6). As the use levels for the catalysts tested were adjusted to match skin-formation times, both SPUR systems showed similar initial reactivity. Subsequently, during the aging process, reactivity drifts, as manifested by changes in skin-formation times, were observed in each test system. Nevertheless, the trends in such reactivity drifts appeared to be consistent across all aged samples. Additionally, no significant changes in cure-through rates were observed for all model systems tested. Assuming that most of the reactivity drift was likely due to the premature curing reactions caused by residual moisture, it is reasonable to conclude that Catalyst V36 appears to have no significant negative impact on reactivity drift in SPUR 1K formulations, similar to industry standard catalysts.
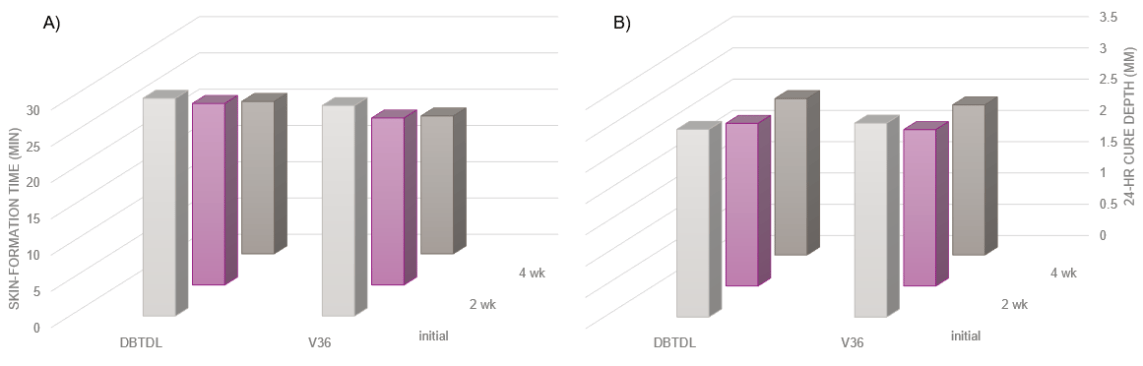
FIGURE 6 ǀ A – Skin-formation time. B – 24-hour cure depth of model trimethoxysilane SPUR system catalyzed with Catalyst V36 and DBTDL over 2-week and 4-week storage time.
Catalyst impact on the color stability of cured SPUR parts was evaluated via QUV accelerated weathering tests. The color changes in ΔE along with QUV exposure times for model trimethoxysilane SPUR system with and without UV additives (0.6% HALS), catalyzed with Catalyst V36 or DBTDL (catalyst-use levels adjusted for similar skin-formation time) are plotted in Figure 7. It is apparent that the inclusion of UV stabilizers in the SPUR formulation significantly improved the color stability of the cured parts. Nevertheless, in both test formulations, samples with either catalyst showed comparable degree of color shift, and hence UV resistance, indicating that Catalyst V36 has similar UV stability to that of the industry-standard catalysts.
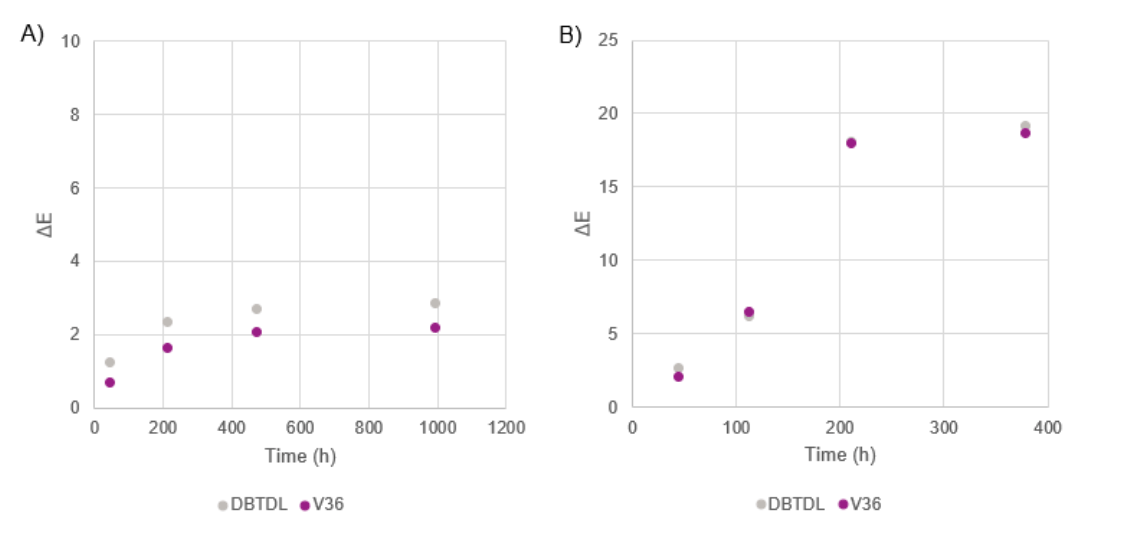
FIGURE 7 ǀ Color changes in ΔE over QUV exposure time for model trimethoxysilane SPUR system catalyzed with Catalyst V36 or DBTDL. A – with 0.6% HALS. B – without HALS.
Conclusion
In summary, we demonstrated that a novel tin-free catalyst is significantly more active than industry standard catalysts in SPUR applications, while providing similar system stability and other end-use properties. Furthermore, we argue that due to its high catalytic efficiency, Catalyst V36 may help enable further adoption of the more environmentally benign ethoxysilane-based SPUR systems, which the current reference catalysts fail to catalyze. The findings generated from the model system may be applicable to wider ranges of SPUR applications. Further research and development will likely provide more opportunities for sustainable and high-performance SPUR materials, contributing to the advancement of PU CASE industries, while addressing environmental concerns.
Acknowledgements
The author would like to thank Albert Bergeman and Steven Robbins, for their technical contributions to the work described in this paper. Jay Patel is also acknowledged for helpful technical discussions.
This paper was originally presented at the 2023 Polyurethanes Technical Conference.
References
1 Karna, N.; Joshi, G. M.; Mhaske, S. T. Progress in Organic Coatings. 2023. 176, 107377-107399.
2 Nomura, Y.; Sato, A.; Sato, S.; Mori, H.; Endo, T. Journal of Polymer Science:Part A, Polymer Chemistry. 2007. 45, 2689–2704.
3 Chi, Q.-C.; Li, J.; Wang, C.-Y.; Ren, Q. Journal of Coatings Technology and Research. 2022. 19(5), 1457-1466.
4 Maudgal, S.; Clair, T. L. International Journal of Adhesion & Adhesives. 1984. 4(3), 129-132.
5 Huang, H. H.; Wilkes, G. L. Polymer, 1989. 30, 2001-2012.
6 Issa, A. A.; Luyt, A. S. Polymers, 2019. 11(3), 537.
7 Okumoto, S.; Fujita, N.; Yamabe, S. Journal of Physical Chemistry A, 1998. 102 (22), 3991-3998.
8Burkhalter, R. S.; Hogue, C. L; Smith, D. L.; Sonner, S. M.; Winningham, M. J.; Yougman, R. E. Journal of Organometallic Chemistry, 2013. 724, 213-224.
This paper may contain copyrighted material, the use of which may not have been specifically authorized by the copyright holder. To the extent this paper contains any such copyrighted material, the material is being used for nonprofit educational purposes reflecting a permitted “fair use” thereof as authorized under Title 17 U.S.C. Section 107. If any copyrighted material included from this paper is further used for purposes that go beyond “fair use,” the copyright holder’s permission is required.