Ready to proof -- Clare 07/7/22
KJ proofed on 7/14
Ready for author
DID YOU KNOW?

How Much Ionic Strength is Too Much?
Video credit: ilyast / Creatas V1ideo, via Getty Images
Did you know that we develop guidelines for relating the colloidal stability of latexes to the ionic strength of the aqueous phase via the “critical coagulation concentration,” (CCC)? In our previous “Did You Know?” column, we included a schematic of the ion concentration around latex particles, which allowed us to mention the “diffuse double layer”. It is that halo around the particles that protects the latex from flocculation and eventual coagulation — we insert those images here for reflection.
Image courtesy of PLOS One, in an article titled “New Perspective in the Formulation and Characterization of Didodecyldimethylammonium Bromide (DMAB) Stabilized Poly(Lactic-co-Glycolic Acid) (PLGA) Nanoparticles.” Authored by Rebecca Gossmann, Klaus Langer and Dennis Mulac. Published: July 6, 2015. https://doi.org/10.1371/journal.pone.0127532
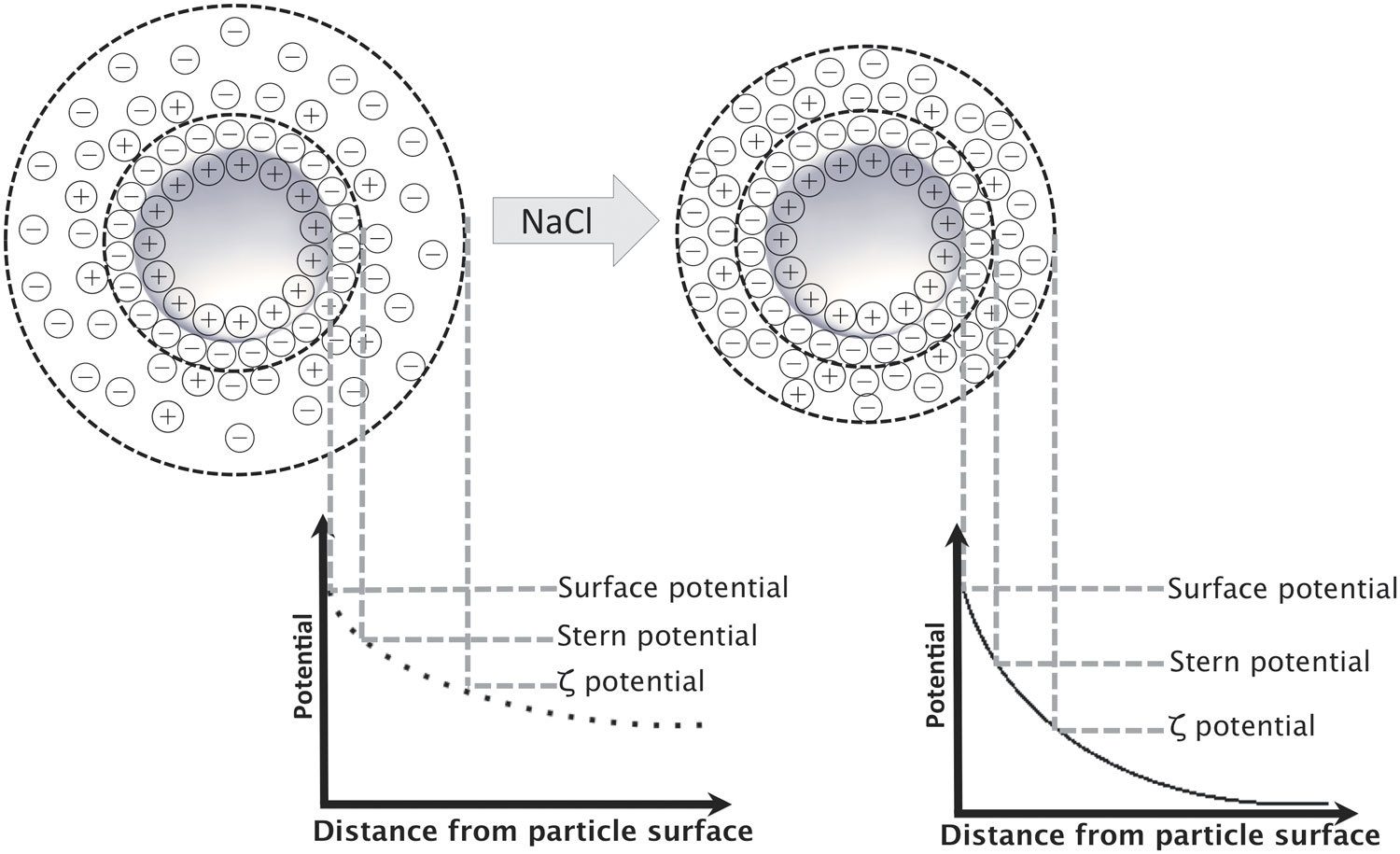
So the question is, “How much ionic strength is too much?” This is quite interesting as it has been fairly common practice over the years to add some monovalent salts to the latex to reduce its viscosity (i.e. reduce the double layer width). But if too much salt is added — disaster! And remember that total ionic strength includes all ionic species including initiator, buffer, surfactant and adventitious metal impurities that may be in your source water.
This brings us back to the CCC — by definition it is the ionic strength at which there is no net repulsive force between the particles as they approach one another. For monovalent ions, the CCC of many latexes is between 0.1-0.2 molar. But beware of the multi-valent ions. The Schulze-Hardy rule states: CCC is proportional to 1.0/Z6, where Z = the cation valency. For mono-, di- and tri-valent ions, the CCC goes like 1/16 : 1/26 : 1/36, or 1/1 : 1/64 : 1/729 respectively. It then becomes quite obvious why di- and (especially) tri-valent cations are of such concern when we want to have an electrostatically stabilized latex. An extension of this concern and to achieve better stability is the role that non-ionic surfactants can play to mitigate such issues.
This subject is treated in more detail in several of our STEP’n workshops. We welcome comments and further discussion of this topic. Please contact us via our website, www.epced.com.

The “Did You Know….?” series is a bi-monthly note from Emulsion Polymers Consulting and Education (EPCEd) that is intended to present simple questions about topics that are important to those working in the emulsion polymers area. Short and concise answers to those questions are presented to educate readers and to elicit comments and further discussion. Some readers will already know the answers and be familiar with the topic, while others, especially those newer to the field, will benefit from the answers and discussion. Experienced practitioners may also find new insights in the discussion. Paint & Coatings Industry magazine has partnered with EPCEd to share the “Did You Know” notes with our readers throughout the year.