Ready to proof — CLJ 11/3/21
KJ sent cocrrection on 11/11
Revised on 11/11
Ready for author on 11/11
FORMULATING WITH MIKE
Introduction to Additives
Part 4
Dispersants
Video: SoraPhotography, Creatas Video+ / Getty Images Plus, via Getty Images
Top Photo:Davizro, iStock / Getty Images Plus, via Getty Images
By Mike Praw, Senior Applications Scientist – Paints, Coatings and Inks, Indorama Ventures: Integrated Oxides and Derivatives, The Woodlands, TX
This is the fourth article in our series on additives, and will cover dispersants and surfactants used to stabilize pigments in coatings. Dispersion is the process where a non-soluble solid is suspended in a liquid and stabilized against settling, separation, agglomeration or aggregation. The concentration of solids in the liquid must be uniform during mixing. Once mixing is removed, the mixture must be stable. For paints and coatings, pigments are being dispersed into a resin/solvent matrix, and stability against settling, flooding, floating or flocculation is required.
Generally, pigments are divided into three main types: primary, extender and specialty. Primary pigments tend to be expensive and give coatings color and hiding. Extender pigments are added to control gloss, lower cost or influence other physical properties. Specialty pigments are added for a specific purpose and include anti-corrosive, effect pigments and nanoparticles. Since primary pigments tend to greatly affect the cost of a coating, using them optimally is necessary.
So why do we disperse pigments? Properties of the coating such as viscosity, tint strength, opacity, dispersant demand and photo stability all depend on the particle size of the pigment (Figure 1). From Table 1 and Figure 1 you notice some properties compete.
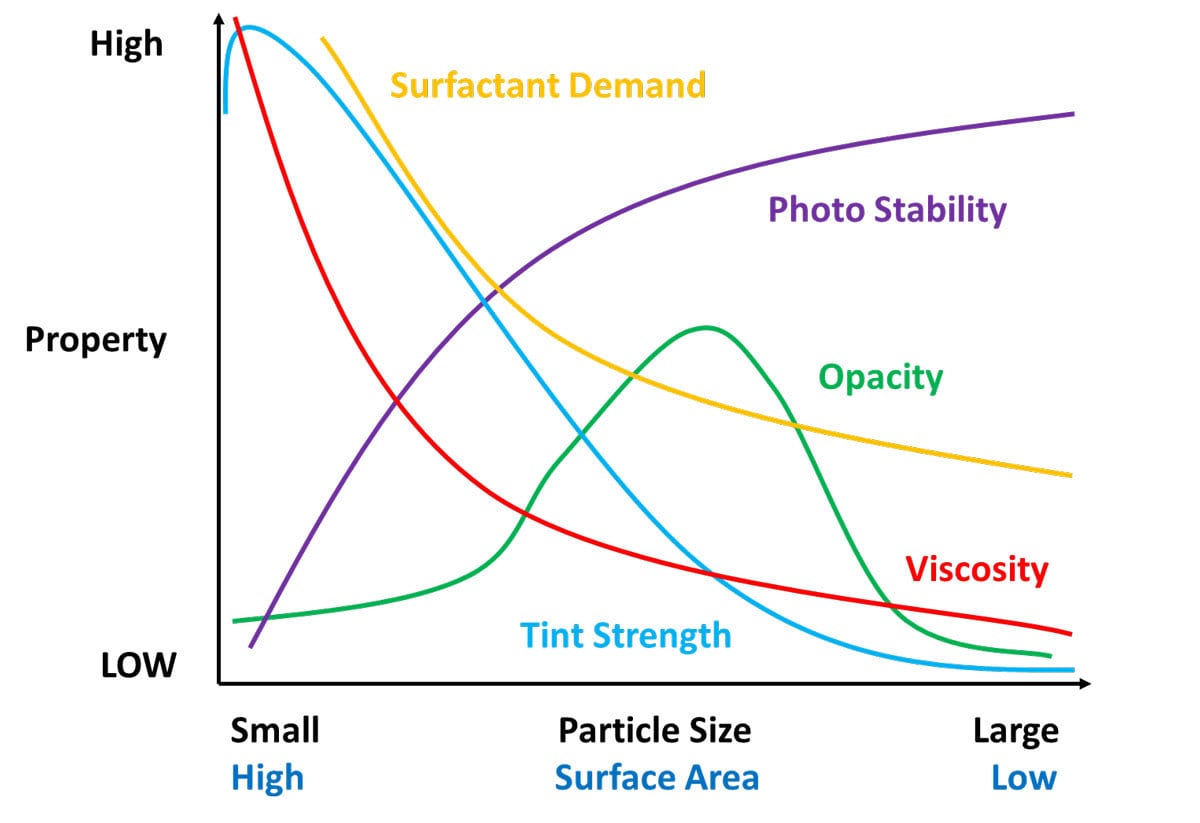
FIGURE 1 ǀ Coatings properties based on pigment particle size.
TABLE 1 ǀ Particle size preferences based on coatings property.
The ultimate particle size for a pigment is determined by the manufacturer and optimized during processing for the right balance of properties. Pigment dispersing occurs by breaking up agglomerations and aggregates; the primary particle size of the pigment should remain unchanged. You are dispersing the pigment by returning it, as close as possible, to its primary size. You are not grinding the pigment or reducing the primary particle size.
Ideally, a good dispersion will break up clumps (aggregates and agglomerates, Figure 2) and will ensure a uniform pigment concentration in the coating. It maximizes hiding power and tint strength while eliminating grit. Figures 3 and 4 highlight the hiding difference between a good and poor dispersion.
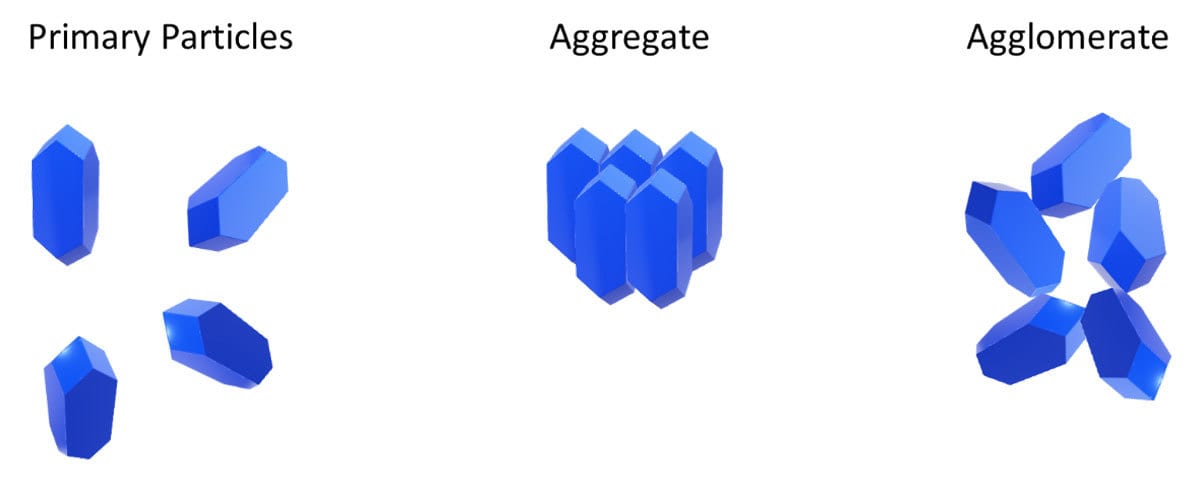
FIGURE 2 ǀ Aggregates and agglomerates.
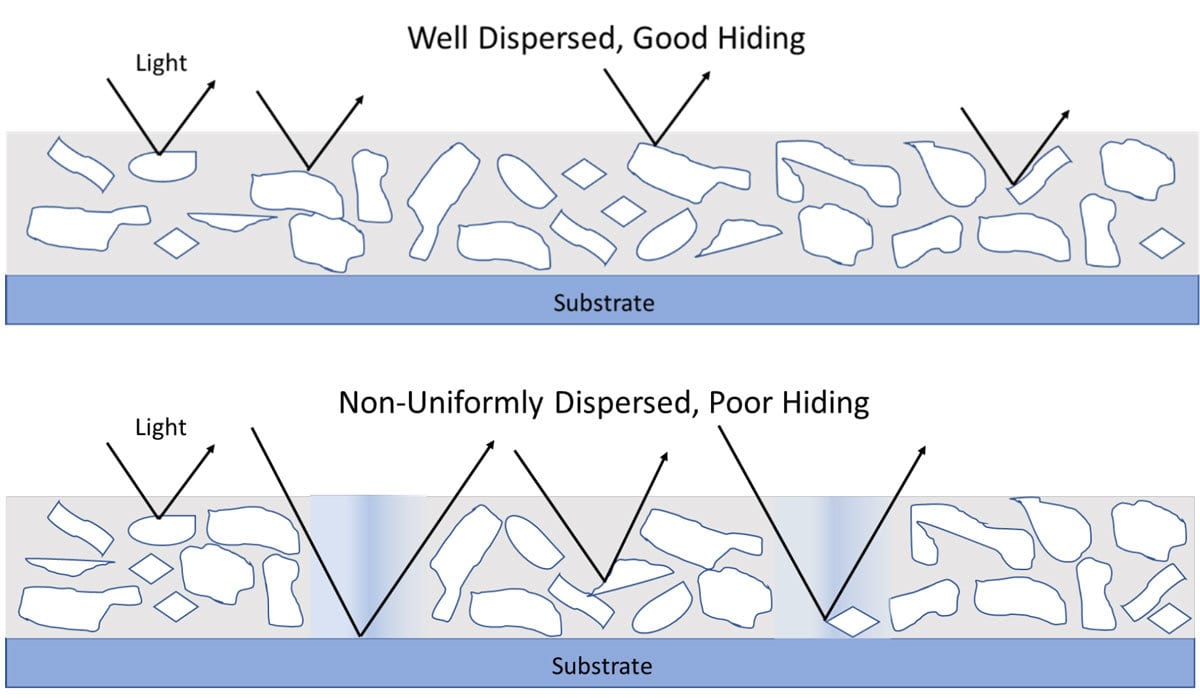
FIGURE 3 ǀ Hiding differences between a good and poor dispersion.

FIGURE 4 ǀ Hiding differences between a good and poor dispersion.
Dispersion Stability
There are several issues related to dispersion stability, which are detailed below.
Settling and Syneresis
Settling takes place when the pigment has a higher density than the liquid media (most cases). The pigments can rise and agglomerate at the surface when the pigment has a lower density than the media. This is rare and generally happens when using hollow spheres or some resin-based pigments. Both settling and rising rates are governed by Stokes’ Law, which states the rate of settling (or rising) is proportional to the particle size and density difference (between the particle and liquid), and inversely proportional to the viscosity. In coatings terms, large, dense pigment particles and a low viscosity will result in rapid settling.
Syneresis is like settling but it is a stratification of liquids in a coating, normally based on gravity. In extreme cases in a water-based paint, enough water will separate out that latex resins will undergo coalescence.
Both settling and syneresis are caused by different compatibilities and densities of liquids in a coating. Changing or increasing the surfactant in a coating to aid in compatibility, and adjusting the rheology package, especially in the low-shear region, will aid in the stability of a uniform coating. Too many formulators will only adjust the rheology package and not completely solve the issue. Optimizing the surfactant package in a coating is critical for good stability. An optimized surfactant package will:
- Stabilize the pigments from re-agglomerating or re-aggregating, as the larger agglomerates or aggregates will settle out faster.
- Cause any settling to be soft and easily re-incorporated.
- Act as a humectant, keeping water within the resin matrix to prevent syneresis.
Figure 5 highlights settling and syneresis.

FIGURE 5 ǀ Settling and syneresis.
Surfactant Stripping
Coatings contain multiple surfactants, and often more than one of each type (pigment dispersants, flow and leveling agents, substrate wetters, emulsion polymerization surfactants, etc.). A white base paint may have a TiO2 dispersant and a colorant acceptance surfactant. Different colorants may each have a different dispersant. This is all well and good if they work together properly. However, often the surfactant from one coating’s raw material will have a greater affinity to another, especially if the latter raw material is surfactant starved. Since formulators do not know which or how much surfactant comes in each raw material, this can lead to a serious issue called surfactant stripping.
For example, we add a colorant to a coating that contains a partially stabilized/surfactant-starved resin emulsion. The surfactant used in the colorant works well and stabilizes the colorant by itself, but when added to the coating, it has a higher affinity to the resin and moves off the pigment, now leaving the pigment improperly stabilized. This is how surfactant stripping operates, i.e. in this case the resin stripped the surfactant off the pigment, but any raw material can strip surfactant of another raw material. Adding a universal stabilizer surfactant will help prevent this. Figure 6 shows this process.
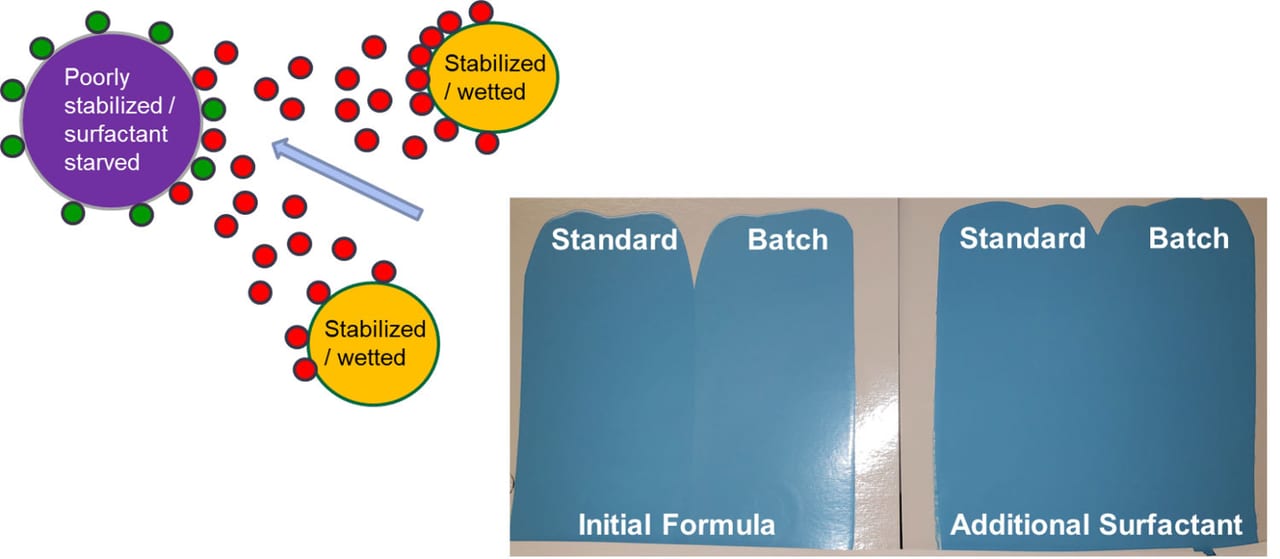
FIGURE 6 ǀ Surfactant stripping.
Flooding and Floating
When a coating is drying and solvent is evaporating, convection currents develop due to concentration gradients of the solvent (water is a solvent, and the use of the word solvent includes water). On a non-porous substrate, solvent concentration will be lowest at the air/coating interface. On a porous substrate, it is more difficult to determine, as solvent is absorbed into the substrate and evaporates at the same time. Since pigments have different densities, particle sizes and affinities to the solvent and resin, their mobility in the coating can be vastly different. These differences can lead to flooding and floating issues.
Floating occurs when there are vertical differences in color, when viewed in the cross section of the coating, and is typified by Bènard Cells. This can lead to silking if the coating does not flow out well and there are irregular coating thicknesses during brush application. Flooding occurs when the different pigments move to the surface at different rates during drying of the coating, as is the case with floating. The difference is the surface tension at the air-liquid interface is low so you obtain a uniform surface color, as opposed to the irregular color of floating, which is different from the coating’s color. Both types of defects lead to differences in color/shade when different levels of shear are applied, such as brush, roller and/or spray (Figures 7-8).
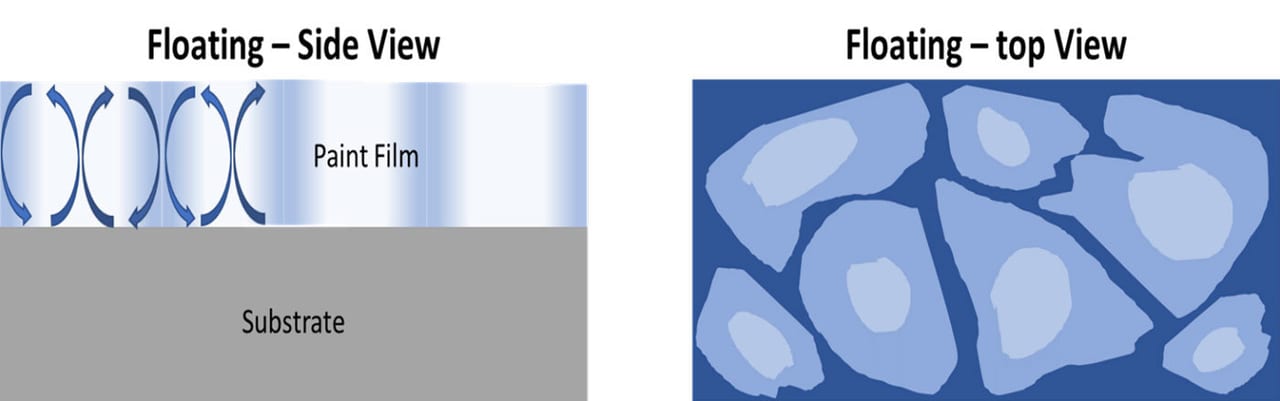
FIGURE 7 ǀ Floating.

FIGURE 8 ǀ Flooding.
Flocculation
Another issue related to dispersion stability is flocculation. Pigments, if not stabilized after the dispersion process, can re-agglomerate or re-aggregate, and this will lead to a loss of hiding, gloss change and many other coating issues. If the destabilized aggregates or agglomerates only contain pigments and surfactants, this is called flocculation. If another raw material acted as a seed for the agglomerate growth, this is called seeding. An example of seeding is pigment being adsorbed on the surface of a resin particle and growing. Figure 9 highlights flocculation and seeding.

FIGURE 9 ǀ Flocculation and seeding in a coating.
Selecting the Right Dispersant
So how are issues like flocculation, settling, flooding and floating solved? We add an agent to help in the dispersion process. These additives are known as dispersing agents or dispersants. The dispersants help in all three stages of the dispersion process:
- They help the liquid wet out the pigment and help remove air trapped on the surface or within agglomerates.
- They then help in the dispersion process by allowing the agglomerates and aggregates to break up easier.
- They help stabilize the dispersion and coating to prevent flocculation, settling, flooding or floating.
- It is obvious that the dispersant choice is critical for an efficient dispersion and a stable, quality paint.
The objectives when selecting a dispersant fall into three catagories:
1. The dispersant can increase the performance of the coating; it will increase color strength and gloss, while also improving compatability and stability.
2. Dispersants are also process aids that help in pigment wetting, decrease dispersion times and help control mill-base viscosity.
3. The last part they play is to affect the coating formulation. Dispersants allow higher pigment loading, improve color stability and can help with rheology control.
So what criteria do you use when selecting dispersants? The most important is the type of pigment being dispersed and what properties you are looking for. Ionic dispersants work best with inorganic primary pigments (titanium dioxide, iron oxides, etc.) and extender pigments. Non-ionic or polymeric dispersants are generally classified by their molecular weight. Low-molecular-weight dispersants are economical solutions for inorganic primary and extender pigments. Medium-molecular-weight dispersants or blends of dispersants give the broadest compatibility with pigments and resins, leading to a more universal system. High-molecular-weight dispersants are used when you require the lowest viscosities, highest color strength and highest gloss. High-molecular-weight dispersants are generally best for expensive organic pigments, as they maximize color development.The higher the molecular weight of the dispersant, the more you will need, as you get fewer molecules per unit weight. You have to balance out the increased cost of higher-molecular-weight dispersants versus the cost of the increased performance of the pigments.
Ionic and non-ionic dispersants use different stabilization mechanisms. Ionic dispersants rely on charge stabilization from the repulsive forces of the electric double layer, known as electrostatic repulsion.
Non-ionic dispersants use the physical repulsive forces of the polymer chains as well as osmotic pressure. The polymer chains attract water through osmosis, which swells the polymer chains and physically prevents particles from getting too close together. This combined effect is called steric repulsion. Steric repulsion is the more robust of the two methods. Some dispersants will combine both stabilization methods for additional performance – this is known as electrosteric stabilization. Figures 10 and 11 demonstrates the different stabilization mechanisms.
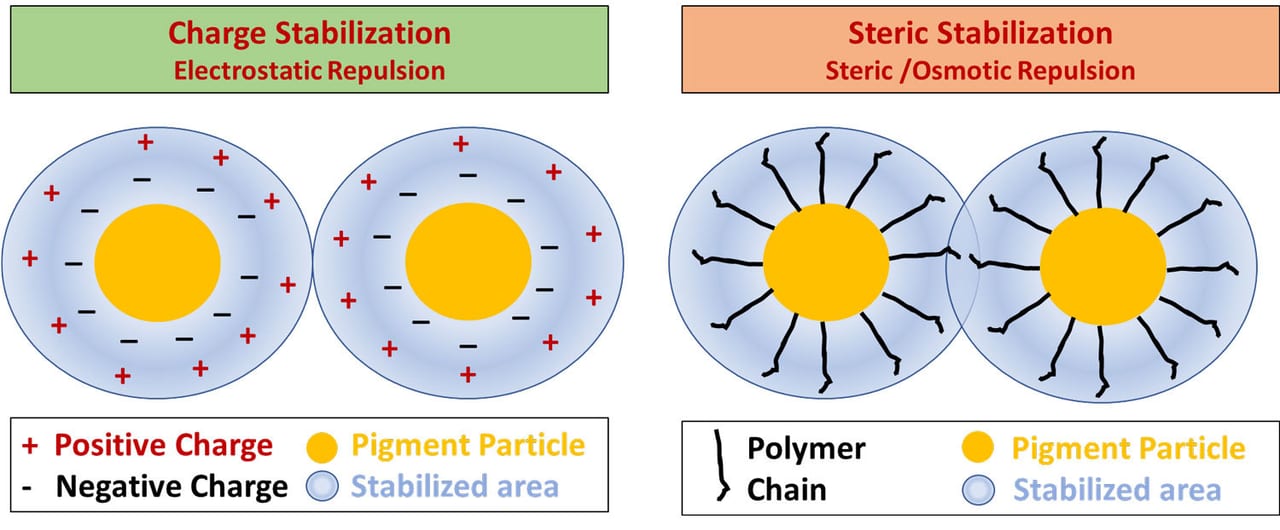
FIGURE 10 ǀ Dispersant stabilization mechanisms: charge versus steric stabilization.
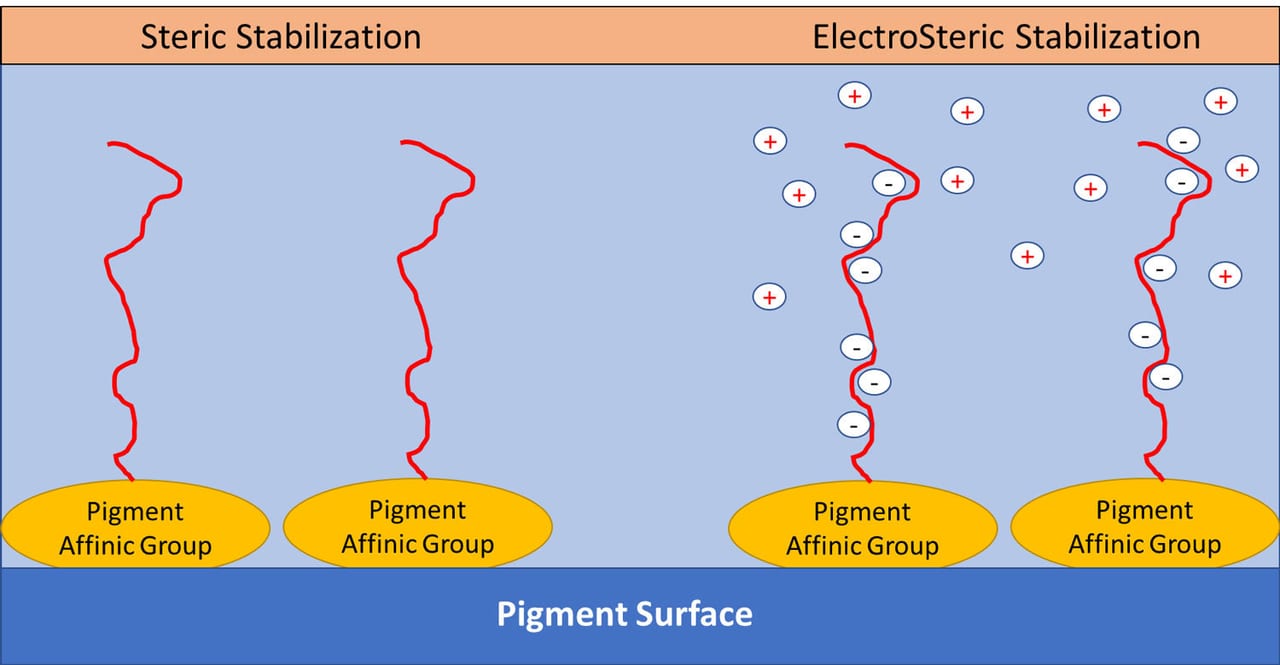
FIGURE 11 ǀ Dispersant stabilization mechanisms: steric versus electrosteric stabilization.
Higher-molecular-weight dispersants stabilize pigments by steric (or osmotic) stabilization. To achieve this, two criteria must be met:
1. The dispersant must strongly adsorb to the pigment; and
2. The dispersant must sufficiently extend out into the solvent or resin phase.
When pigment particles approach each other, a concentration gradient forms as the polymer chains become more concentrated between the pigments. This increases the osmotic pressure, so solvent moves into the region to lower the concentration gradient. This leads to the pigment particles being forced back apart and they are prevented from flocculating.
So, which is better? It really depends on the coating and what you are trying to achieve, but overall, a combination of charge and steric stabilization, and low to medium molecular weight will give you the best performance in a coating, especially in base/colorant systems.
Determining the Best Dispersant Level
Like any other additive, more is not better. In fact, performance will decrease once past the ideal level. To determine the best dispersant and approximate the ideal dispersant level, a design of experiment will be the most efficient tool. However, once the dispersant is identified, a ladder study is best to determine the optimum level needed. The standard method is to measure mill base viscosity with increasing dispersant level, and plot it. Figure 12 shows the determination of dosage level of a dispersant.
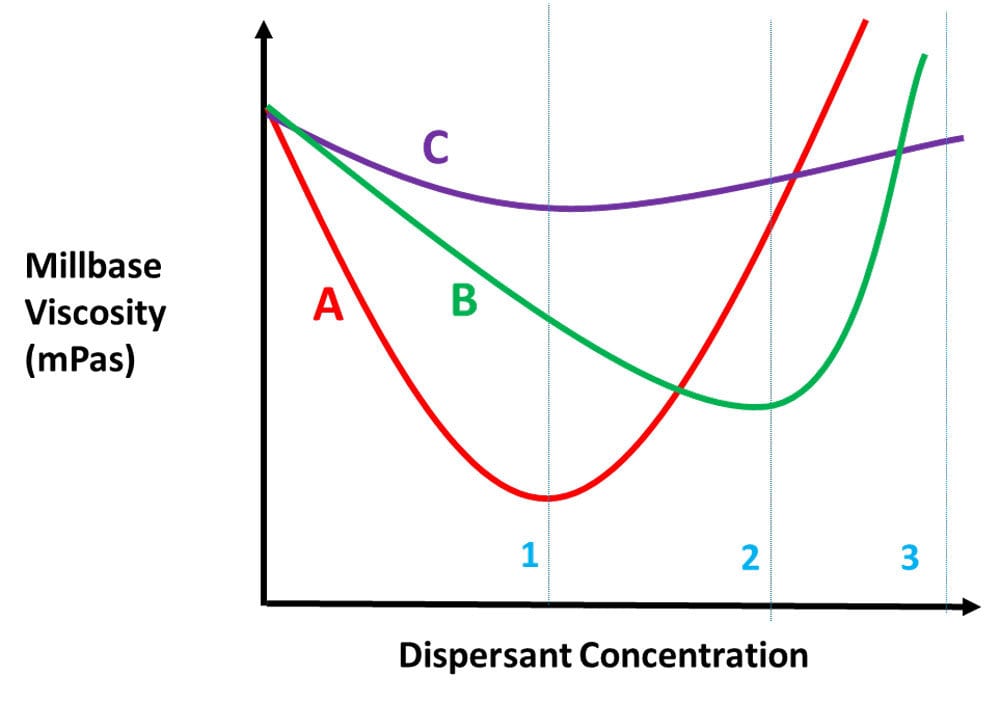
FIGURE 12 ǀ Determination of the dosage level of a dispersant.
The key is not to formulate on a pinhead. In Figure 12, we see that the red curve has the lowest viscosity and is the preferred dispersant, but its curve has a narrower plateau than the green or purple curves. At dispersant concentration 1, the red dispersant is the obvious choice. It uses less dispersant and has the lowest viscosity. But what if the concentration change between 1 and 3 is so small your production process cannot consistantly weigh out the dispersant accurately within the range of the curve? Then the purple curve would be better. While the purple curve is the flattest of the three curves, in almost all areas the green curve has a lower viscosity and is preferred over the purple curve if the red dispersant cannot be used.
So why lower viscosity? As you increase the level of dispersant in the mill base, viscosity drops to the optimum level, then as you increase the dispersant level, dispersant/dispersant interactions will increase viscosity and make dispersion more difficult.
Pigments vary in their hydrophilic/hydrophobic nature and because of this, so do dispersants. The Hydrophilic/Lipophilic Balance (HLB) of a surfactant goes up to 20, with 20 being completely water soluble. The lower the number the more hydrophobic (lipophilic) it is. Most pigment dispersants have hydrophobic and hydrophilic portions. In aqueous systems the hydrophobic region attaches to the pigment and the hydrophilic region extends into the water phase. The opposite is true for solvent and 100%-solids based systems. Because of this, choosing the correct structure and HLB of the dispersant is critical to a stable dispersion. For example, a dispersant for titanium dioxide and calcium carbonate in an aqueous system was dispersed with both a hydrophobic and hydrophilic dispersant (high and low HLB). As expected, a more hydrophilic dispersant worked the best (lower levels and quicker dispersion).
Often water sensitivity is a concern in coatings for either scrub or corrosion resistance. While this may lead you to consider a hydrophobic surfactant, you will need more of it and it may not give optimum opacity, gloss and tint strength. Often a hydrophobic surfactant will remain in the resin matrix, resulting in long-term water sensitivity, while a hydrophilic surfactant may leach out quickly giving better long-term water resistance.
One other type of dispersion mechanism is controlled flocculation. In this method, the dispersant attaches to both the pigment and itself to form a three-dimensional structure that stabilizes the dispersion. The advantage can be increased stability, but you need significantly more dispersant that can create other issues in a coating. Figure 13 shows this ad hoc network.
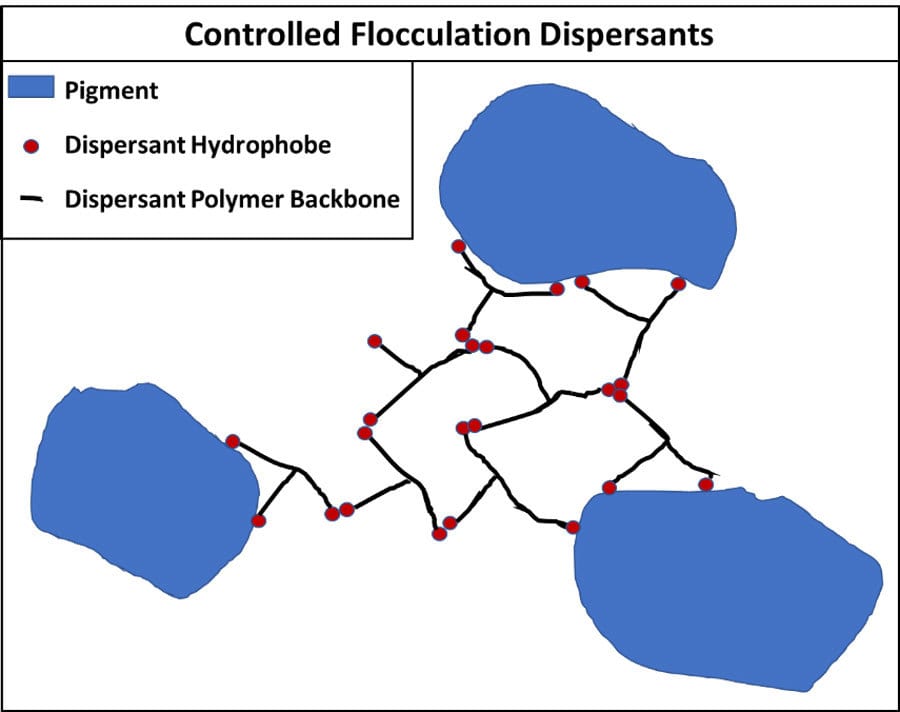
FIGURE 13 ǀ Controlled flocculation dispersants.
Polymeric dispersants are made with different chemistries, with urethane, acrylic and polyester being predominant. However, the increased use of renewable/green chemistries has changed how we look at dispersants.
Conclusion
There are many options to determine which dispersant to use and how to optimize the system. Overall, you want a dispersant that yields a robust system that is easy to formulate, and optimizes the desired properties with the minimum cost. You need the proper dispersant because even quality dispersions can become a flocculated mess if the system is not stable. A good dispersion is the most important factor in making a high-quality coating. It takes planning and preparation, but it is easily obtainable.
While optimizing the use of additives is time consuming, it is worthwhile since you will have a more robust coating often at a lower price. The thing to remember is more is not better when it comes to additives.
For information, email Michael.Praw@us.indorama.net.
*All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
