Ready to proof – CLJ 11/5/21
KJ sent new figures to Clare on 11/11.
Revised on 11/11
Ready for author on 11/11
Photo: Bim, iStock/Getty Images Plus, via Getty Images
Patent-Pending Coatings Technology
Increases Accessibility to Environmentally Friendly Paint
By Samantha Moran, R&D Group Leader; and Cynthia Baricos, R&D Director, Sheboygan Paint Company
Drivers to improve the environmental footprint of coatings have been present since the 1960s, and customers are increasingly demanding high-performance, green coating technologies. Enabling technology developed in the marketplace to meet these demands has primarily revolved around reducing the volatile organic compound content, eliminating hazardous air pollutants and similar strategies. These incremental improvements, while impactful, are not significant enough to enable customers to truly operate in a manner that is better for the environment and kinder to employees. Isocyanates and formaldehyde are objectionable ingredients in coatings but have been considered necessary evils in producing a high-quality finish of superior performance. Recent innovations have brought new isocyanate/formaldehyde-free technology to the marketplace; however, customers have been unable to transition to this chemistry in the general industrial market due its lack of adhesion to metal substrates. This was the state of the industry until today.
Sheboygan Paint Company has developed a patent-pending technology that bridges this gap. The new innovation enables isocyanate/formaldehyde-free technology to be used not only in metal applications, it also proves to be highly efficacious over wood and a wide variety of primers. Products catalyzed with this unique catalyst will still have hours of pot life, while curing quickly, and exhibiting superior corrosion and chemical resistance properties. In an environment of increasingly strict regulations, we anticipate the utility of this invention to span multiple markets, displacing conventional solventborne technologies.
The Chemistry
The existing coating technology is based on a Michael addition reaction between a malonate functional resin and an acrylate resin that is catalyzed with a strong base, tetrabutylammonium. When the strong base is added to the resinous mixture, the base catalyzes the reaction by deprotonating the donor resin (malonated resin). This then allows the acrylate resin to make a carbon-carbon bond on the malonate’s methylene group. The catalyst is then consumed by the reaction and releases byproducts: carbon dioxide and ethanol. This coating rapidly forms hard, durable films; however, it has poor adhesion to metal substrates and some primers.
Sheboygan Paint Company has developed a catalyst that not only reacts with the resin but also interacts with metal substrates. Our R&D team found that triphenylphosphine (TPP) catalyzes the Michael addition reaction while also promoting adhesion to metal substrates. Triphenylphosphine has a lone pair of electrons that attack electrophilic alkenes like acrylates (Figure 1). In the case of a Michael addition reaction, this catalytic mechanism starts the reaction from the acrylate molecule instead of through the strong base. The triphenylphosphine molecule attacks the electrophilic acrylate group, i.e., α, β-unsaturated carbonyl compound, and forms a conjugate base of phosphonium anion. This conjugate base is strong enough to deprotonate the hydrogen from the acidic methylene group on the malonate. After deprotonation, the acrylate forms a carbon-carbon bond with the malonate. During this reaction, TPP is not consumed and instead stays in the coating system. Because of its lone pairs, TPP has an extremely high affinity to metal and proceeds to form a metal ligand when the coating is applied over metal substrates. Not only does the TPP catalyze the reaction, it also acts as an adhesion promotor. The TPP chemically binds to the metal substrate and has a physical entanglement to the coating, giving the product excellent adhesion over metal.
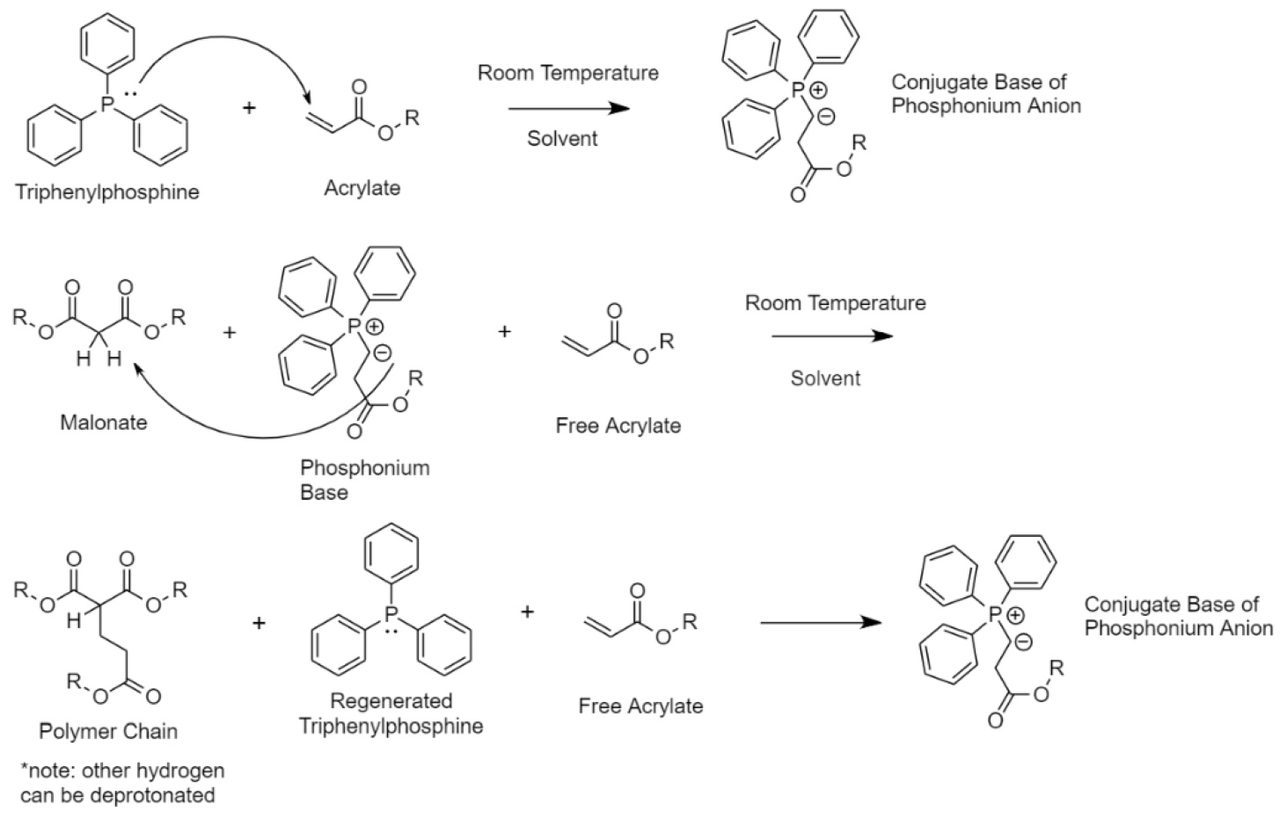
FIGURE 1 ǀ TPP reaction mechanism.
The team also explored improving the adhesion of this new technology over primed surfaces. Many primer systems contain acidic compounds, interrupting the new technology’s Michael addition reaction. The current catalyst technology uses tetrabutylammonium that is blocked with bicarbonate blends. When this catalyst is used over certain 2K primers, their performance properties are hindered. This is due to the blocking of either the primer or topcoat curing components. Testing shows that primed systems perform worse in corrosion protection and adhesion performance when the strong base is used in the topcoat. With the use of TPP catalyst, these results are not seen. The coating is able to adhere to a variety of primers without hindering the cure response, performance or adhesion. Although it is not providing adhesion to the primed surface, TPP does not inhibit the primer from creating a protective barrier.
Our TPP catalyst is currently in the provisional patent phase, and the R&D team has extensively studied this catalyst’s performance versus other Michael addition initiators. This study evaluated two coating formulation types with different malonate and acrylate resins, along with a variety of catalysts. The catalysts tested were: sodium hydroxide in ethanol, potassium hydroxide in ethanol, 1,4 diazabicyclo octane (DABCO), tri o-tolylphosphine, trioctylphosphine, tricyclohexylphosphine, triphenylphosphine and tetrabutylammonium blends. These catalysts were also tested at various levels to determine the minimum and maximum dosage.
Each catalyst was tested at various levels in both coating Formulas 1 and 2. Six out of the eight catalysts were eliminated from further substrate testing. Sodium hydroxide, potassium hydroxide, DABCO, tri o-tolylphosphine and tricyclohexylphosphine did not cure the film or they created a spontaneous, non-uniform reaction where the coating would not form a complete film. Trioctylphosphine reacted differently from the previous catalysts and had rapid catalytic action, which created a non-uniform, spontaneous grit formation throughout the film. Catalysts triphenylphosphine and tetrabutylammonium blends cured the film completely and uniformly in both formulation types, at various catalyst levels. These two catalysts were then studied further to determine if their addition contributes to the coating’s adhesion to a substrate.
Testing Methods and Results
Triphenylphosphine and tetrabutylammonium catalysts were tested in both coating Formulas 1 and 2 at 2%, 3.5% and 5% levels. These coatings were then applied by a conventional spray gun onto various substrates. The substrates tested were: ground-cold rolled steel, smooth-cold rolled steel, thin steel, iron phosphate-treated steel, chrome-treated aluminum and galvannealed steel. Films were then allowed to cure for seven days before being tested. The following tests were then conducted: adhesion (ASTM D3359 Method B), mandrel bend (ASTM D522), MEK solvent rubs (ASTM D5402), gloss (ASTM D523), pencil hardness (ASTM D3363) and impact resistance (ASTM D2794).
The supporting charts (Figures 2 and 3) exhibit a clear benefit to adhesion over a wide range of substrates when using TPP as a catalyst. Superior adhesion is seen over chrome-treated aluminum, thin steel, iron phosphate-treated steel and ground-cold rolled steel when using TPP. When using tetrabutylammonium, fair adhesion is seen to iron phosphate-treated steel, and no adhesion is seen to any other substrates. Similar results were achieved with both formulation types when using TPP. Poor adhesion is seen when tetrabutylammonium is used in Formula 1 versus slightly better adhesion in Formula 2. A ladder study was conducted to see if the level of catalyst played a role substrate adhesion. The level of TPP does not affect the coating's ability to have adhesion. With regards to the tetrabutylammonium catalyst, a higher level of catalyst worsens the coating's ability to adhere to the substrate.
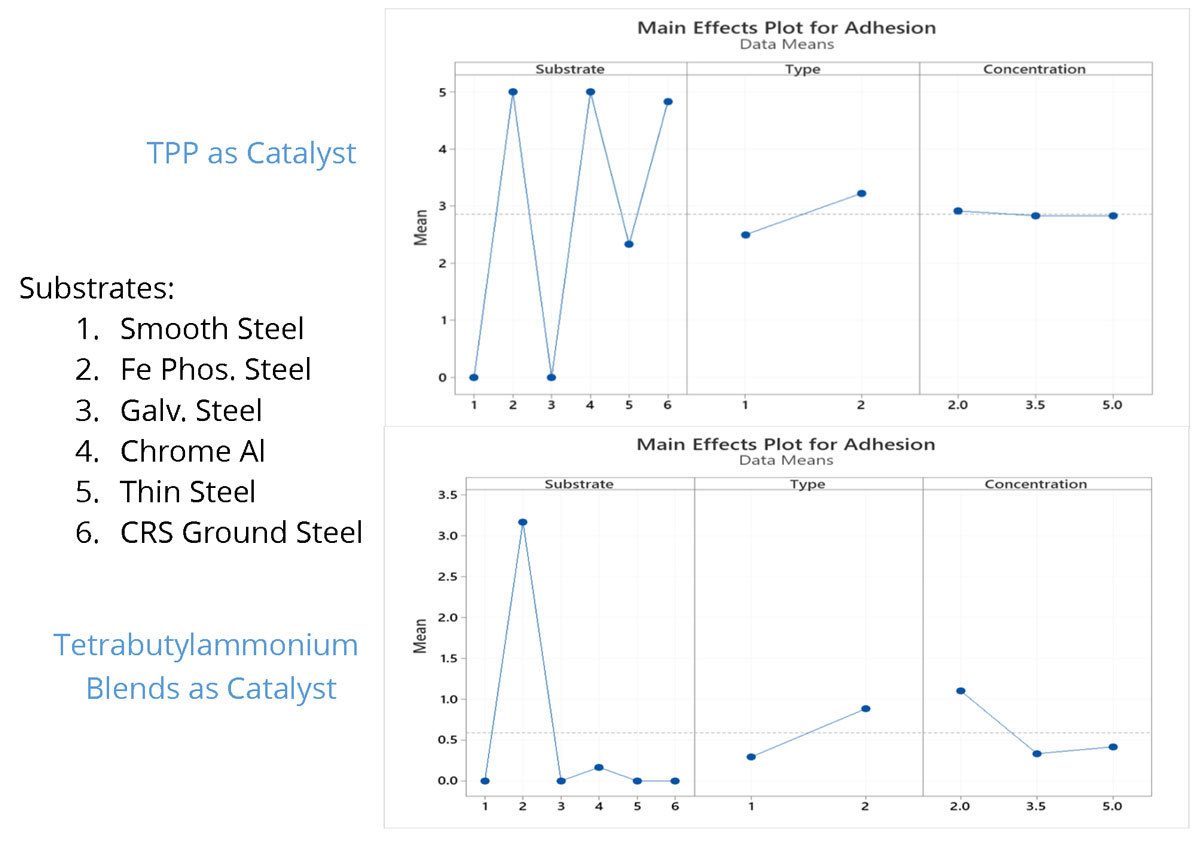
FIGURE 2 ǀ Main effect plots for adhesion using TPP or tetrabutylammonium blends.

FIGURE 3 ǀ Adhesion test results.
The use of TPP displays high, consistent MEK solvent rubs (100+) across all types of substrates, all formulation and concentrations. When using tetrabutylammonium catalyst, 90 rubs are achieved over most substrates, except for galvannealed steel. If tetrabutylammonium is used in a formulation over galvannealed steel at a 2% level, the coating will not cure. The catalyst is completely blocked by the zinc-treated panel. The cure response is different over each substrate, in each formulation, and at various concentrations. This shows an inconsistency in the cure response of the coating when using the tetrabutylammonium-based catalyst.
The coating’s gloss is not typically affected by the type of catalyst chosen. The only case where we see a low gloss is with the under-cured formulation that uses tetrabutylammonium as a catalyst over galvannealed steel. When using TPP versus tetrabutylammonium, harder films are produced over all substrates. Lower pencil hardness is seen when either catalyst is used over smooth steel and galvannealed steel. Across both catalysts, Formula 1 presents harder film when compared to Formula 2. The catalyst concentration does not seem to play a major role in film hardness.
The coatings are able to generate a higher level of impact resistance when TPP is used versus tetrabutylammonium. Tetrabutylammonium produces weaker films over all substrates, but it is most notable over smooth steel, galvannealed steel, and thin steel. Formula 1 produces more impact-resistant films over Formula 2 when either catalyst is used. The level of TPP does not affect the impact resistance. A higher level of tetrabutylammonium produces weaker films when compared to a lower catalyst dosage.
Both TPP and tetrabutylammonium perform well on the mandrel bend test over iron phosphate-treated steel, galvannealed steel, chrome-treated aluminum and thin steel. When TPP is used over smooth steel, the coating loses its flexibility and shows signs of stress cracking. The same thing is seen when using tetrabutylammonium over both smooth steel and ground cold rolled steel. With the use of TPP, Formula 1 is more flexible than its use in Formula 2. The results are consistent across both formulation types with the use of tetrabutylammonium. A large decrease in flexibility is seen with both catalysts when they are used at high concentrations of 5%.
Invitation to a Greener Future
There are many uncertainties in this day and age, however one thing is certain. The future is demanding that we treat the earth and each other better. For industrial coatings this means that we must innovate faster and better to develop commercially viable solutions to meet this market need. Sheboygan Paint Company is thrilled to unveil this new patent-pending catalyst technology – the company’s first in its 100 years in operation. This catalyst technology is expected to disrupt the marketplace because it enables the general industrial sector, including general metal and industrial wood, to adopt isocyanate- and formaldehyde-free technology. The R&D team invites other industry innovation leaders to collaborate on ways to implement and improve our industry’s sustainable impact. Let’s work together toward a greener future!
To receive a free, full whitepaper detailing the development, testing and results of this
patent-pending technology, please contact author Samantha Moran. We look forward to the opportunity to collaborate and improve environmentally friendly options in the coatings industry!
For more information, e-mail smoran@shebpaint.com or cbaricos@shebpaint.com.
