Ready to proof -- Clare 12/8/21
KJ proofed on 12/10
Ready for author
Video: olsjon, Creatas Video+/Getty Images Plus, via Getty Images
Sustainable Waterborne Acrylic Polymer Dispersions
By Dr. Shanti Swarup, Ph.D., Adjunct Professor, Department of Coatings and Polymeric Materials, North Dakota State University, Fargo, ND; PPG Collegium Member
Waterborne acrylic dispersions are widely used in the production of coating formulations for industrial applications such as automotive, decorative and marine coatings. These dispersions are classified in two broad categories: 1. Low molecular weight (weight average molecular weight, Mw <50,000 Dalton) and 2. High molecular weight (weight average molecular weight, Mw >50,000).
Low-Mw Polymers
Low-Mw polymer dispersions are traditionally made in a water-compatible organic solvent. The solvent is heated to its boiling point in the flask, into which a co-monomers feed containing acid-functional acrylic or methacrylic monomer is added simultaneously with the initiator dissolved in organic solvent over 2-5 hours. Upon completion of the polymerization as judged by consumption of all the monomers, acid groups are partially or completely neutralized with base, and the polymer thus formed is diluted with water. This type of polymer in coatings will release solvent in the atmosphere during application and curing, contributing to the volatile organic compounds (VOCs). If the solvent is distilled out from the polymer it will generate undesirable waste.
To overcome VOC and waste generation issues, low-Mw acrylic polymer dispersions can be made by using a reactive solvent such as 1,2 epoxy hexane, 1,2 epoxy cyclohexane, 1,2 epoxy octane, or epoxy ester of neodecanoic acid (known as Cardura E™, Figure 1). These solvents can be heated to their boiling point or to 150-160 °C in case of Cardura E (boiling point 251-271 °C) in the flask into which co-monomers and initiator dissolved in the reactive solvent can be added simultaneously over 3-5 hours. One of the co-monomers in the monomer feed will need to be an acid-functional acrylic or methacrylic when epoxy is used as a solvent. During the addition of the monomer feed, two reactions will take place: 1. acid groups will react with epoxy groups of the solvent, generating hydroxyl groups and; 2. all the acrylate and methacrylate monomers will be polymerized by the free radicals generated from the dissociation of the initiator (Figure 2). All the reactive solvent will be grafted onto the acrylic polymer backbone, leaving no free solvent after completion of the monomer polymerization. In this process, acid groups will need to be more than the epoxy groups such that after consumption of all the epoxy, 2-10% acid groups are left. These leftover acid groups are partially or completely neutralized with the base such as ammonia, diethyl methanol amine or the likes and then diluted with water. The final solid of these dispersions can be 25-50%, viscosity <1,000 centipoise, particle size 50-200 nanometers, Mw 2,000-40,000 (US Patents: 6881786, 10975259).
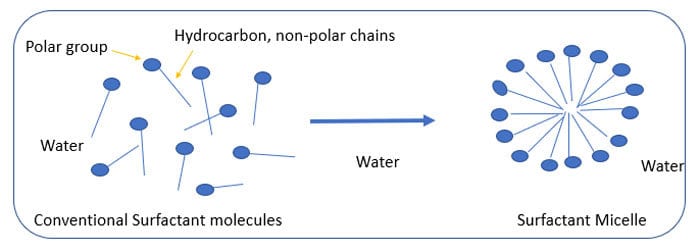
FIGURE 1 ǀ Structure of of Cardura E, where R1 plus R2 = 7 carbon atoms, viscosity at 23 °C is 7 milli-Pascal/second.
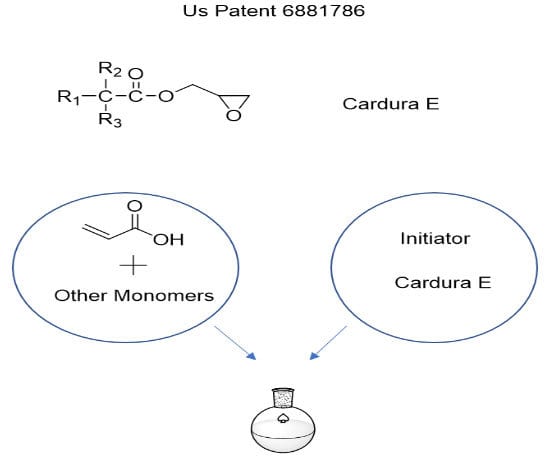
FIGURE 2 ǀ Reaction scheme. The flask contains Cardura E.
Control of Particle Size
The particle size can be controlled by controlling the amount of acid groups and their degree of neutralization, the higher the acid and base, the lower the particle size. Usually, an acid value of 15-30 on the solid is sufficient to have a stable dispersion. At low acid value, 100% neutralization of acid with base is required. At higher acid value, less than the 100% is needed to control the viscosity. The higher the degree of neutralization, the greater the viscosity.
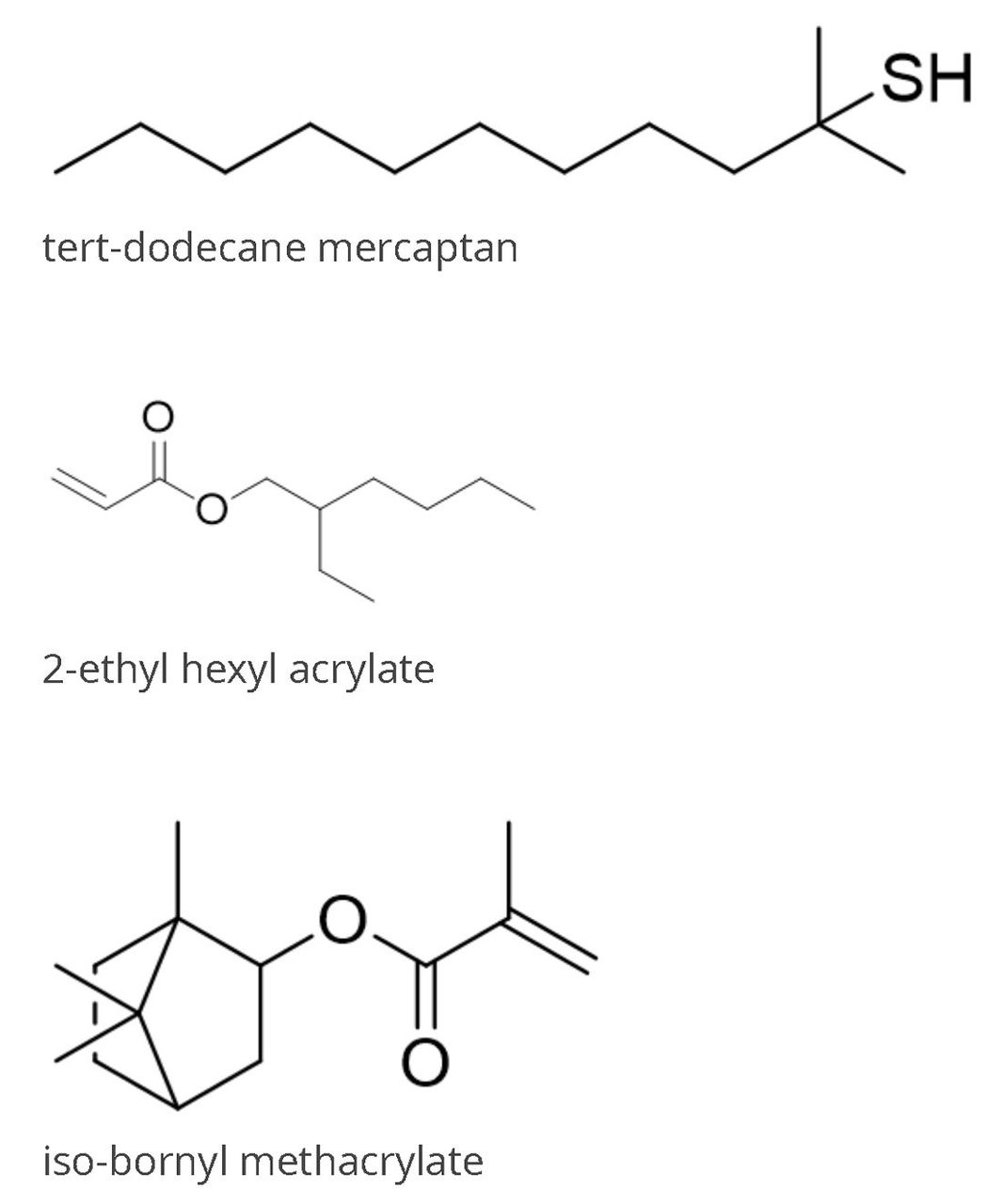
FIGURE 3 ǀ Structures of tertiary dodecyl mercaptan, 2-ethyl hexyl acrylate (EHA), isobornyl methacrylate (IBoMA). H atom on EHA and IBOMA attached to tertiary carbon can get abstracted at 150 °C.
Control of Mw
The reaction temperature, amount and types of free-radical-forming initiator, chain-terminating monomers, and viscosity all play a role in determining the final Mw. At higher temperature, more free radicals are formed, creating faster chain propagation and lower Mw. The higher amount of initiator will also form more radicals, lowering Mw.
Continuous formation of radicals is necessary because the time of existence of a typical radical chain is short, usually less than 10 seconds; consequently, new chains must be started regularly. If the half-life of an initiator is too short to provide the necessary supply of radicals during the entire reaction, either an initiator with a longer half-life can be used, or the initiator can be added to the reaction mixture continuously during the reaction. The chain transfer monomers, which contain abstractable hydrogen at the reaction temperature, can be used to terminate the chain propagation and thus lower the Mw. The examples of chain transfer monomers are: tertiary dodecyl mercaptan, 2-ethyl hexyl acrylate, or isobornyl acrylate or methacrylate (Figure 3).
The low-Mw, cationic polymer dispersion can also be made by using amine functional monomers such as aminopropyl acrylate or N-methyl aminopropyl acrylate in the monomer feed. Upon the completion of the polymerization, tertiary amine thus formed can be neutralized with acids such as acetic acid, lactic acid or sulfamic acid.
These acrylic dispersions can be used to grind pigments and enhance flow and leveling to improve appearance, stain resistance and other coating properties.
Furthermore, the low-Mw core:shell acrylic dispersions can also be made using this method. Thus, after making slightly acid-functional polymer in epoxy solvent, a second feed of comonomers containing 10-30% acrylic or methacrylic acid and initiator can be added and copolymerized, optionally functional monomers such as hydroxyethyl acrylate or methacrylate can be used to crosslink with the crosslinker in the coating. The degree of neutralization with base can be adjusted to have a viscosity less than 1,000 cps before or after dispersion in water (UP Patent: 8242211). The final solid could be ~45%. The core to shell ratio can be manipulated to control the rheology (low shear and high shear) during application and eventually coating properties, specifically flip-flop (brightness and darkness) in metallic colors. Usually, core is more hydrophobic than shell and is around 70-80%.
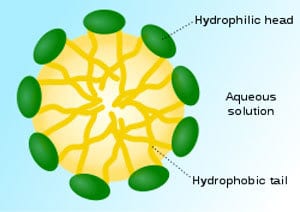
FIGURE 4 ǀ Structure of a surfactant micelle, where hydrocarbon chains are oriented inwards and hydrophilic polar (green) groups are at the surface.
High-Mw Polymers
High-Mw (>50,000) polymers are prepared directly in water using a process called emulsion polymerization. This process involves free radical polymerization that usually starts by making an emulsion of water, monomer and surfactant. The most common type of emulsion polymerization is an oil-in-water type emulsion, in which droplets of monomer are emulsified with the surfactant in a continuous phase of water. The amount of surfactant used must be more than its CMC (critical micellar concentration). This is the surfactant concentration at which micelle (Figure 4) starts to form, usually in the concentration range of 0.5-3%. The acrylic monomers are emulsified in these micelles and water-soluble initiator such as ammonium persulfate is used as a source of radicles.
After heating the water-containing surfactant to 75-85 °C, emulsified monomer feed and initiator are added simultaneously over 2-5 hours. A small number of monomers are solubilized in the water phase, which are polymerized by free radicals forming oligomeric radicals that migrate to the monomer droplets stabilized by surfactants where chain propagation takes place (Figure 5). The process continues until all the monomers are consumed and the growing chain terminates. At this point, the monomer-swollen micelle has turned into a polymer particle. More monomer from the droplets diffuses to the growing particle, where more initiators will react. Eventually the free monomer droplets disappear, and all remaining monomer is located in the particles and surfactant micelles will disappear. The surfactant molecules will locate at the particle surface and will stabilize each particle by electrostatic repulsion (in case of ionic surfactant) or by stearic stabilization (in case of non-ionic surfactant). The final product is a dispersion of polymer particles. The final solid of these dispersions can be 25-50%, viscosity <500 centipoise, particle size 50-200 nanometer, and molecular weight can be 50,000 million.
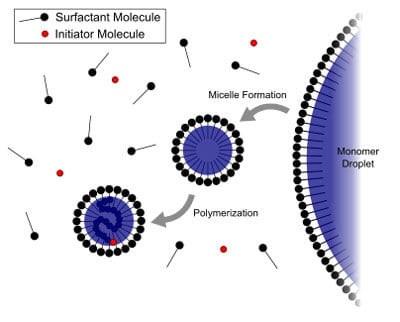
FIGURE 5 ǀ Scheme of emulsion polymerization.
Control of Particle Size
The particle size can be controlled by controlling the amount of surfactant — the higher the amount of surfactant, the higher the number of micelles and therefore, the lower the particle size. In some cases, acid-functional monomers can be used on the polymer backbone, which can also lower the particle size after neutralization. The amount of surfactant in the system should be kept to a level less than 3%, as these molecules over time will absorb water in the coated film, causing film defects such as cracking and corrosion.
Control of Mw
Molecular weight of the polymer will depend upon the choice of monomers used. Chain-transferring monomers, such as 2-ethyl hexyl acrylate and iso bornyl acrylate, will lower the molecular weight, and so will mercaptans. Use of di-acrylate/methacrylate such as hexanediol diacrylate, ethylene glycol di-methacrylate or tri-acrylate such as tri-acrylate of trimethalol propane will increase the molecular weight in the range of millions. The diacrylate can be used in the amount up to 70% without changing the viscosity of the system as the polymerization takes place inside the surfactant micelles that are stabilized by the ionic charge at the surface or steric stabilization in the case of non-ionic surfactants, or both. However, ionic stabilization is much more efficient for long-term polymer stability than steric stabilization.
Similar to low-Mw polymers, acrylic polymers of high Mw can be made in core-shell structure particles consisting of polymers of differing hydrophobicity, core being hydrophobic (virtually free of acid) and shell being hydrophilic. Shell is made hydrophilic by co-polymerizing 20-50% acrylic acid or methacrylic acid and 10-20% hydroxy functional monomers, and the rest being non-functional. pH of the reaction during synthesis is kept in the range 4-6, to keep low viscosity.
Thus, sustainable low-Mw and high-Mw polymers can be made without generating any waste during polymer synthesis, using reactive solvents and water as a reaction media, which will help reduce VOCs of coating formulations.
For more information, e-mail shanti.swarup@ndsu.edu.
