Improving Indoor Air Quality
with Amino Alcohols

Credit: cloudytronics / iStock via Getty Images Plus

Credit: x / x via Getty Images Plus
By Mark Langille, Advancion Corporation, Buffalo Grove, IL; and Romain Severac, Advancion Corporation, Argenteuil, France
Driven by environmental, health and safety concerns, coating technologies have undergone a dramatic shift in the past few decades. Most of this movement has been driven by the reduction of VOCs and other hazardous materials in coatings formulations (Figure 1). The continuous development and improvement of waterborne technologies has enabled many solvent-based systems to be replaced with waterborne chemistries that contain significantly lower VOC content than their solvent-based counterparts. VOC levels have been pushed even lower by the development of low-VOC and zero-VOC water-based formulations. A continuation of this trend emerging in the global coatings industry has been focused on the development of functional coatings that not only limit emissions of VOCs into the environment but extract and remove VOCs that have originated from other sources.1,2 A functional coating with VOC-remediation capability could improve indoor air quality and provide a means to scavenge VOC emissions from sources that have proven to be more challenging to address.
FIGURE 1–ǀ–Scheme depicting the evolution of more environmentally friendly paints and the emergence of VOC-remediation technologies.
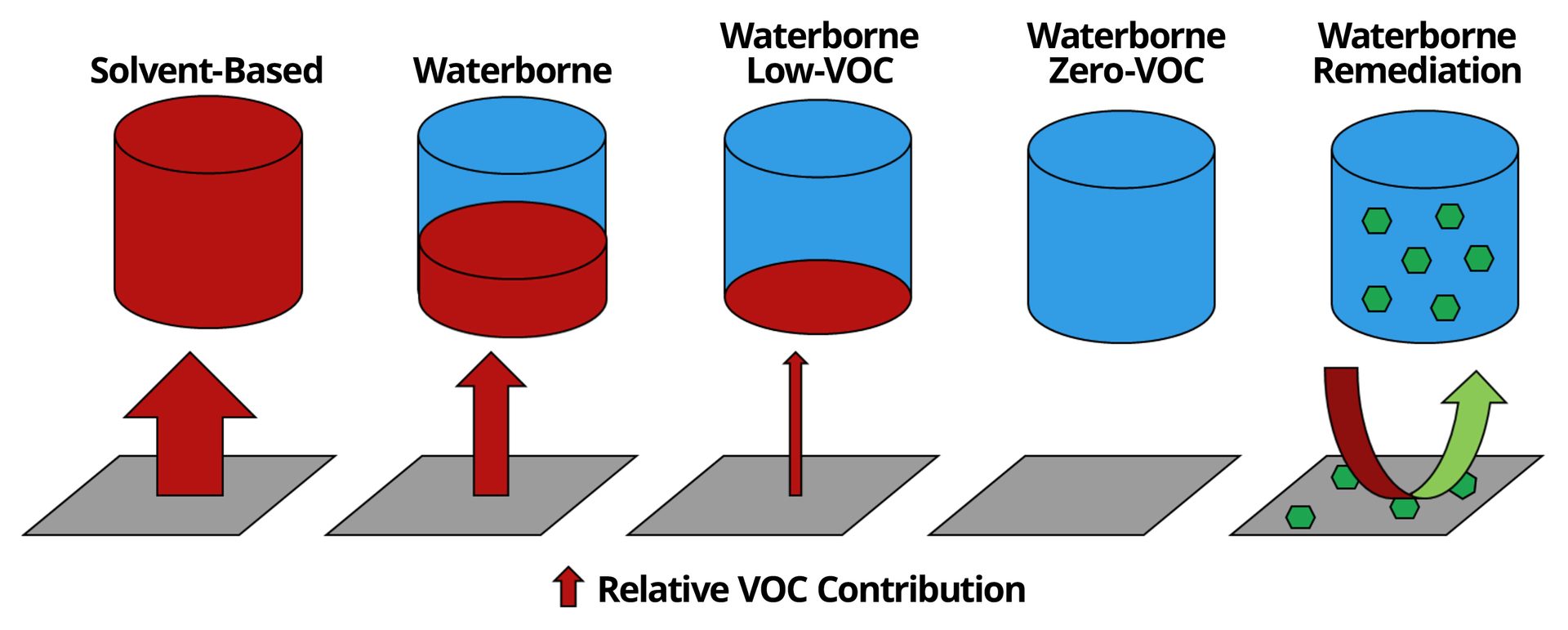
Surveys of air quality point to a large discrepancy between the quality of air found indoors versus outdoors. Concentrations of pollutants are found to be significantly higher in indoor air than outdoor air.3 Among the most prevalent VOCs found in indoor air, aldehydes account for three of the four found at highest concentrations, with formaldehyde being the most prevalent.4 Formaldehyde therefore makes for a good target candidate for indoor air remediation efforts. Formaldehyde poses serious health risks associated with respiratory issues, asthma symptoms and carcinogenicity.5 The U.S. Environmental Protection Agency (EPA) considers formaldehyde a probable human carcinogen,6 while the U.S. National Toxicology Program lists formaldehyde as known to be a human carcinogen.7 Sources of formaldehyde are numerous and include furniture, cabinetry, flooring, carpets, electronics, household cleaning products, and open stoves and heaters. Although formaldehyde emissions from certain product categories are facing increasingly stringent formaldehyde-emissions requirements, such as composite wood products,8 approaches to reduce formaldehyde levels by targeting reductions from each individual source presents a significant challenge. A more attractive solution would be to engineer remediation technologies into existing building materials that are commonly used within indoor environments.
In this paper, we show how amino alcohols based on Advancion chemistry possess unique scavenging functionality that is ideally suited for the remediation of airborne formaldehyde. These materials are easily incorporated into waterborne formulations to create functional coatings capable of improving indoor air quality. Furthermore, this formaldehyde-scavenging technology is effective at low use levels and easily formulated into coatings, making it readily accessible to today’s formulators.
The industry and our supply chain partners, which include paint and coatings professionals, have some of the most stringent compliance requirements in the United States.
Experimental Methods
Static-Airflow Formaldehyde-Scavenging Test
Formaldehyde-scavenging tests were conducted under static-airflow conditions for amino alcohol compounds. For each sample, testing was conducted by weighing approximately 0.10 g of scavenger material onto a small watch glass. The watch glass containing the scavenger was placed into a clean, dry, 1-liter glass bottle. Then 10 µL of 18.5% aqueous formaldehyde was transferred into a glass vial and placed into the jars, which were subsequently sealed with caps having a port for sampling with Dräger tubes. The jars, each with a different formaldehyde scavenger, were allowed to equilibrate under ambient laboratory conditions for four days. The jars were then sampled for free formaldehyde in the vapor phase by inserting a Dräger tube through the port and withdrawing the headspace gas. During sampling, the headspace is continuously replenished by ambient air, causing a dilution effect. The reported results were adjusted accordingly to account for this dilution effect. If the initial Dräger tube chosen was outside the range of the formaldehyde being measured, a second Dräger tube was used to obtain results within the appropriate calibration range.
Dynamic-Airflow Formaldehyde-Scavenging Test by ISO 16000-23
Dynamic-airflow formaldehyde-scavenging tests were performed by Eurofins Scientific according to the ISO 16000-23 test method, “Indoor air — Part 23: Performance test for evaluating the reduction of formaldehyde and other carbonyl compounds concentrations by sorptive building materials.” Paint samples were first homogenized prior to brush application onto a glass plate in two coats, each at a spread rate of 140 g/m² and dried for 8 hours between coats. The coated glass panels were then placed into individual 119-liter controlled-temperature and humidity chambers. The airflow rate into the chamber yielded an air change rate of 50% chamber volumes per hour. The ratio between the coating surface and the chamber volume was set up at 1.4, corresponding to both walls and ceiling coating conditions. The air supplied to the chamber contained 100 µg/m³ formaldehyde (equivalent to 80 ppb) at 23 ± 1 °C and 50 ± 3% relative humidity. As a reference, the World Health Organization (WHO) considers an airborne formaldehyde concentration at or above 100 µg/m³ to be unsafe,9,10 so the coatings in these studies were exposed to a continuous supply of airborne formaldehyde just at the WHO-recommended exposure threshold. To reach a steady state in the chamber, the protocol was extended from a maximum of 28 days to 42 days.
Results
The amino alcohols discussed in this work consist of primary amines bonded to tertiary carbons and include 2-amino-2-methyl-1-propanol (also known as aminomethyl propanol or AMP), 2-amino-2-ethyl-1,3-propanediol (also known as aminomethyl propanediol or AEPD), and tris(hydroxymethyl)aminomethane (also known as tromethamine or Tris) (Figure 2). The variations in structure and hydroxyl functionality among these molecules yield materials of different physical properties (Table 1). For instance, pure AMP is a gel-like crystalline solid at room temperature, having the lowest boiling point, highest vapor pressure and strongest basicity of the three amino alcohols that were evaluated. Commercially, AMP is commonly supplied as a 95% solution in water (AMP-95™) to aid in handling by lowering viscosity and depressing the freezing point. Pure AEPD is a thick, viscous liquid with an intermediate boiling point, vapor pressure and basicity. AEPD is commercially supplied as an 85% solution in water (AEPD™ VOX 1000) for ease of handling. Tris (supplied as TRIS AMINO™ Crystals), on the other hand, is a white crystalline solid with a boiling point over 300 °C and has the mildest basicity.
FIGURE 2–ǀ–Chemical structures of AMP, AEPD and Tris.
AMP: 2-amino-2-methyl-1-propanol

REVERSE PINCH TO ZOOM
AEPD: 2-amino-2-ethyl-1,3-propanediol

REVERSE PINCH TO ZOOM
Tris: tris (hydroxymethyl) aminomethane

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
TABLE 1–ǀ–Physical properties of AMP, AEPD and Tris.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
Amino alcohols like AMP and AEPD are commonly used in coatings applications for their multifunctional properties, which arise from a multitude of interactions these materials have with other formulation ingredients. The interaction between the amino alcohols’ amino group and negative charges on pigment particle surfaces makes them ideal co-dispersants to maximize optical and mechanical properties.11,12 The amino group can also interact with negative charges found on dispersing agents, resins and other polymers to provide stability to pH and rheology. These materials can also enhance the biostability and shelf life of formulations through a synergistic interaction between amino alcohols and registered biocides. These same materials can either participate in or inhibit certain chemical reactions that are advantageous to formulators. For example, the chemical bond between the nitrogen and tertiary carbon is extremely stable to UV irradiation, which prevents degradation and yellowing, and avoids the formation of peroxy radicals. This is a critical feature that enabled the VOC-exemption status of AMP by the U.S. EPA and the Government of Canada, as peroxy radicals can lead to the generation of ground-level ozone.13
Here we show that amino alcohols can participate in chemical reactions with formaldehyde and that AEPD, and Tris can react with airborne formaldehyde to behave as formaldehyde scavengers. The reaction between amino alcohols and formaldehyde still occurs even when these materials are formulated into a waterborne architectural coating.
Amino alcohols of the appropriate composition can readily react with formaldehyde in aqueous or organic solution to form stable oxazolidines through a ring-closing mechanism. While this reaction is reversible, with the oxazolidine product capable of reversibly releasing formaldehyde and the amino alcohol, this only occurs under extreme conditions and is highly dependent on the specific amino alcohol and oxazolidine structures. The oxazolidine product is essentially inert under typical indoor environmental conditions. Reaction schemes of AMP, AEPD and Tris with formaldehyde are shown in Figures 3, 4 and 5, respectively. AMP can react with one equivalent of formaldehyde to yield an oxazolidine, while both AEPD and Tris can react with two equivalents of formaldehyde to yield bisoxazolidines.
FIGURE 3–ǀ–Reaction of AMP with one equivalent of formaldehyde to yield an oxazolidine.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
FIGURE 4–ǀ–Reaction of AEPD with two equivalents of formaldehyde to yield a bisoxazolidine.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
FIGURE 5–ǀ–Reaction of Tris with two equivalents of formaldehyde to yield a bisoxazolidine.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
It was not until recently that these materials were confirmed to have reactivity to airborne formaldehyde. This was demonstrated by evaluating amino alcohols in a relatively simple, static-airflow formaldehyde-scavenging test as described in the experimental section. The data show that amino alcohols can be effective scavengers of formaldehyde, even in the absence of a solvent (Table 2). The control, which contained an initial source of formaldehyde but no formaldehyde scavenger, was found to have an airborne formaldehyde concentration of 945 ppm after a four-day equilibration time. For the sealed bottles containing 0.1 g of AMP and Tris, the airborne formaldehyde concentration was measured as 70.8 and <0.1 ppm, respectively. Tris was found to have a similar formaldehyde-scavenging potential as sodium bisulfite in this test, a known formaldehyde scavenger in the industry. However, sodium bisulfite can evolve noxious SO2 fumes upon atmospheric exposure, which significantly limits its utility in many applications.
TABLE 2–ǀ–Scavenging of airborne formaldehyde under static-airflow conditions.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
The static-airflow test showed that amino alcohols can scavenge airborne formaldehyde. However, this test is not a very good representation of indoor conditions where formaldehyde might be encountered. Typical levels of formaldehyde under modeled indoor conditions can be studied by the ISO 16000-23 test method, which is a dynamic-airflow test that can quantify the efficiency of formaldehyde-scavenging technologies under controlled conditions.
For amino alcohols to function as an efficient formaldehyde scavenger in a coating or construction material application, they must remain active and chemically available for reaction with formaldehyde in the final formulation. This was evaluated by formulating AEPD and Tris into an architectural coating formulation (Table 3). A set of architectural paints were prepared containing either no formaldehyde scavenger, 0.5% AEPD or 0.5% Tris on total formulation weight. Both materials were added to the let-down. Tris was first prepared as a 40% solution in deionized water prior to addition to the paint during the let-down, while AEPD was added as supplied.
TABLE 3–ǀ–Model architectural coating formulation.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
Results from the ISO 16000-23 test method are reported as a percent consumption of formaldehyde versus days of exposure in the chamber. Air containing 100 µg/m³ formaldehyde is continually introduced to the chamber at a rate of 0.5 chamber volumes per hour. The air at the chamber inlet and outlet is sampled for quantification of airborne formaldehyde. The percent scavenging efficiency of formaldehyde is calculated as a ratio between the airborne formaldehyde concentration at the chamber outlet divided by the concentration at the chamber inlet under steady-state conditions.
The percent formaldehyde consumption for the three paint samples is plotted over a 42-day testing period and shows dramatically different results between paint samples (Figure 6). The control, which does not contain a formaldehyde scavenger, has a formaldehyde-scavenging efficiency of 0–10%, which can generally be considered within the error of measurement. On the other hand, AEPD has a high initial scavenging efficiency of formaldehyde at 60%, which decreases to 20% after 42 days. Tris has the highest formaldehyde-scavenging efficiency, initially at 75% and decreasing to 50% after the 42-day testing period. These results provide strong evidence that amino alcohols like AEPD and Tris are still chemically active toward formaldehyde even when formulated into a waterborne coating. These coatings, therefore, have the ability to remove and scavenge formaldehyde from the environment through the use of low levels of amino alcohols.
FIGURE 6–ǀ–Formaldehyde-scavenging efficiency over time for coatings with no scavenger, 0.5% AEPD or 0.5% Tris.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
An ideal formaldehyde-scavenging technology should be easy to implement and have minimal effect on other performance properties of the coating. Based on these results, both Tris and AEPD are found to be highly effective formaldehyde scavengers, even at low concentrations. Evaluations of the effect these additives have on coating properties are ongoing, but initial results indicate that there is minimal impact to most properties. This additional work will be shared in future publications.
Conclusions
With the use of amino alcohols based on Advancion technologies, it is possible to create functional coatings to improve indoor air quality. Amino alcohols such as AEPD and Tris are easily formulated into waterborne coatings and are highly effective at low use levels as scavengers of airborne formaldehyde. Of the two amino alcohol chemistries evaluated under dynamic testing conditions, Tris had superior formaldehyde-scavenging potential, but AEPD can offer additional functionality as it not only scavenges formaldehyde but can also adjust solution pH, disperse pigments and boost the performance of registered biocides.10,11 These chemistries provide an easily accessible pathway to advance the evolution of environmentally friendly coatings by creating functionality that improves indoor air quality within homes, schools, offices and other buildings.
This paper was presented at the 2025 Waterborne Symposium in New Orleans.
References
1 Day, N.; Olsen, J.; Thieves, J.; Polliack, A. Improving Indoor Air Quality Using a Smart Additive. Paint & Coatings Industry 2018, 34 (3), 36–40.
2 Xu, J.; Zou, J.; Cui, W.; Yang, W.; Doll, P.; Bohling, J. Formaldehyde Abatement Functional Latex for Interior Coatings. Polymers Paint Colour Journal & Asia Pacific Coatings Journal 2019, October, 15–17.
3 Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chemical Reviews 2010, 110, 2536–2572.
4 Kirchner, S.; Derbez, M.; Duboudin, C.; Elias, P.; Gregoire, A.; Leaute, C.; Lucas, J.-P.; Mandin, C.; Pigeon, M.; Riberon, J.; et al. Indoor Air Quality in French Dwellings. Air Infiltration and Ventilation Centre, AIVC-CR12, 2009.
5 Golden, R. Identifying an Indoor Air Exposure Limit for Formaldehyde Considering Both Irritation and Cancer Hazards. Critical Reviews in Toxicology 2011, 41 (8), 672–721.
6 United States Environmental Protection Agency. Formaldehyde. (accessed April 2025).
7 United States National Toxicology Program. Report on Carcinogens, Fourteenth Edition – Formaldehyde. (accessed April 2025).
8 U.S. Code of Federal Regulations. 40 CFR Part 770. Formaldehyde Emission Standards for Composite Wood Products.
9 World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, 2010. (accessed April 2025).
10 Nielsen, G. D.; Larsen, S. T.; Wolkoff, P. Re-evaluation of the WHO (2010) Formaldehyde Indoor Air Quality Guideline for Cancer Risk Assessment. Archives of Toxicology 2017, 91, 35–61.
11 Severac, R.; Fernandes, Y. Painting a More Stable Picture. European Coatings Journal 2018, 6, 18–21.
12 Severac, R.; Fernandes, Y. Improving Pigment Dispersion and Paint Stability with Versatile Amino Alcohols. Coatings World 2019, September, 132–135.
13 Troester, L.; Brutto, P.; Peera, A. The Journey to VOC Exemption. Paint & Coatings Industry 2015, January.
