Ready to proof -- Clare 4-9-21
KJ proofed and pdf sent to Clare on 4/12
CLJ revised on 4/12
CLJ revised on 4/13
CLJ revised on 4/14
FORMULATING WITH MIKE
Introduction to Additives
Part 1
Video: SoraPhotography, Creatas Video+ / Getty Images Plus, via Getty Images
Top Photo:Davizro, iStock / Getty Images Plus, via Getty Images
By Mike Praw, Senior Applications Scientist – Paints, Coatings and Inks,
Indorama Ventures: Integrated Oxides and Derivatives, The Woodlands, TX
This will be the first of four articles on additives. This article will offer an introduction to additives, as well as details about rheology modifiers and suspension agents. Other articles in this series will cover foam-control agents, surface tension modifiers, and finally surfactants and pigment dispersants. Future articles will also cover solvents, resins, pigments, and other coatings raw materials.
Additive Use in Coatings
Additives are used in small quantities and can have the highest per unit cost of coatings raw materials. They are used to facilitate production or to improve certain properties of the wet coating or the final film. They are absolutely essential in paint; however, more is not better. In fact, with many additives higher levels can hurt performance.
Some additive types are:
Rheology modifiers and suspension agents;
Defoamers and air-release agents;
Flow and substrate wetting agents;
Driers and catalysts;
Pigment dispersants;
Bugicides: (fungicides, biocides, preservatives, etc.);
Surface slip/anti-mar agents: (waxes, silicones, etc.);
UV protection agents (UV absorbers, hindered amine light stabilizers, nanoparticles, etc.);
Non-pigment anti-corrosives (pigment anti-corrosives will be covered in the pigment article);
Plasticizers (plasticizers used for film formation will be covered in the solvents article).
A general rule of thumb is that additives should make up less than 2% of the final formula for the coating (by weight). If you are higher than this, it is best to re-evaluate your formula. It is more important to choose the correct additives initially rather than adding more additives to fix problems, contrary to what additive companies may advise you to do.
A common formulation misstep is to add an additive to fix a problem, which creates another problem. You then use another additive, which creates a different issue, and the cycle continues, leading to the “Additive Death Spiral.” For example, you add a substrate wetting agent, which stabilizes macro foam, then you add a defoamer to combat the macro foam, and you get surface defects. Now you add a leveling agent for the surface defects and stabilize micro foam, leading to the need for a de-aerator. Next the de-aerator interferes with the rheological package, raising viscosity. This now prevents the coating from wetting out, leading to the need for more substrate wetting agents. As you can see, you have to understand all interactions to select the correct additive package to minimize cost and maximize performance. The biggest trap coatings formulators fall into is having a favorite additive package and using it everywhere. What works well in one system may be marginal in another system. Every system is unique and needs its own additives, otherwise you would just use a paint you have already formulated.
Rheology Modifiers and Suspension Agents
Rheology Modifiers
While commonly combined in the same category, rheology modifiers and suspension agents have two very different purposes. Rheology modifiers are used to adjust the viscosity, flow, leveling and sag resistance of the coating to optimize the coating for a particular application method. They influence film thickness and appearance, mixing, film formation and settling. Common water-based rheology modifiers include cellulosic, urethane (HEUR), alkali-swellable (ASE, HASE), clays (kaolin, bentonite, attapulgite), waxes and fumed silicas. They work in different ways: associations (depends a lot on interactions with surfactants or resins), volume filling, house of cards, etc.
Rheology is the science of the flow of matter. Viscosity, which is only one part of rheology, is the resistance of a fluid to deformation by shear, elongation or tensile stress. Coatings can behave either as a Newtonian, dilatant or pseudoplastic liquid. In a Newtonian liquid the viscosity remains the same, irrespective of the shear that is applied. In a dilatant or shear-thickening liquid, the viscosity increases with increasing shear. In a pseudoplastic or shear-thinning liquid, the viscosity decreases with increasing shear. I will discuss thixotropy, which is a special form of a pseudoplastic liquid, later in this article.
Figure 1 illustrates the differences in viscosity with varying shear. Note that a liquid’s behavior is only over a specified viscosity range. All pseudoplastic coatings have a no-shear, high-viscosity point. As shear rate increases, the viscosity decreases until the high-shear plateau is reached. This is the range in which viscosity should be recorded. Once shear increases past the high-shear plateau, viscosity increases due to the movement of solid particles (resin, pigment, etc.) in a non-laminar flow system, in effect becoming dilatant. Figure 2 illustrates these properties.
In coatings formulation, pseudoplastic behavior is most common. Dilatant behavior is sometimes observed in pigment dispersions and should be avoided. Dilatant behavior is highly dependent on the concentration of particles. Almost any dispersion of hard particles will exhibit dilatant behavior at a high enough concentration. Solvent-based coatings tend to be more Newtonian (less pseudoplastic) than water-based systems because solvent evaporation increases viscosity after application, preventing sag and drips. No true Newtonian coating system is possible. Solvent-based clearcoats can be approximately Newtonian, but will exhibit a slight viscosity reduction at high shear. See Figure 1 for Newtonian, dilatant and pseudoplastic behavior.
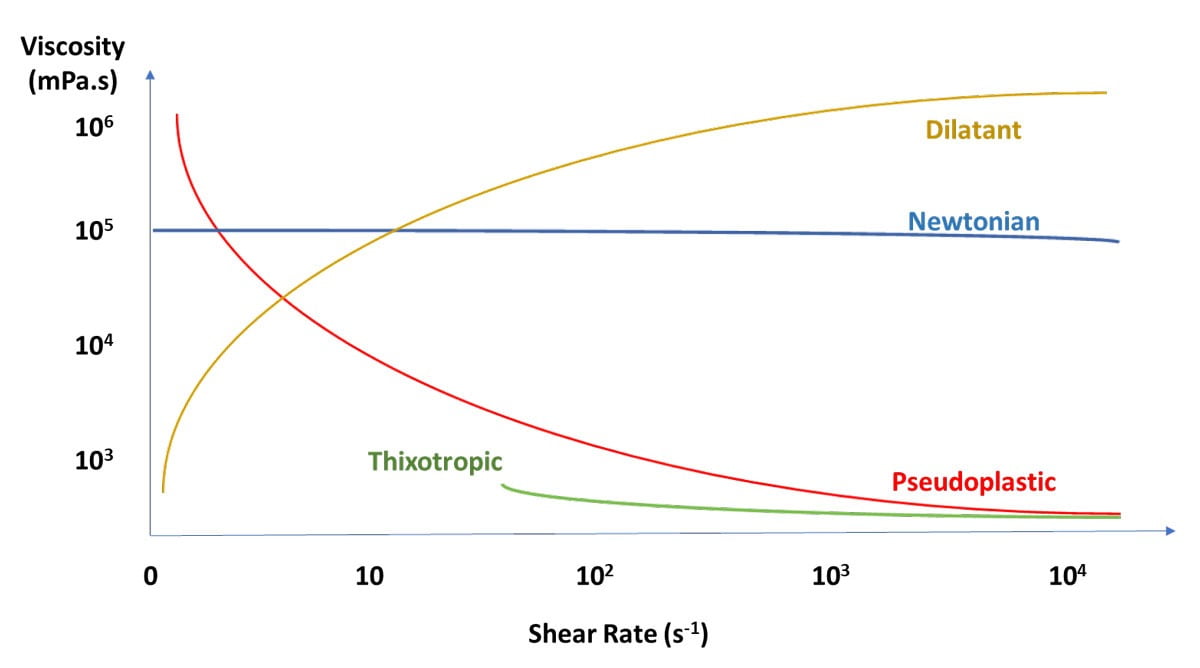
FIGURE 1 ǀ Viscosity versus shear rate: Newtonian, dilatant, pseudoplastic and thixotropic liquids.
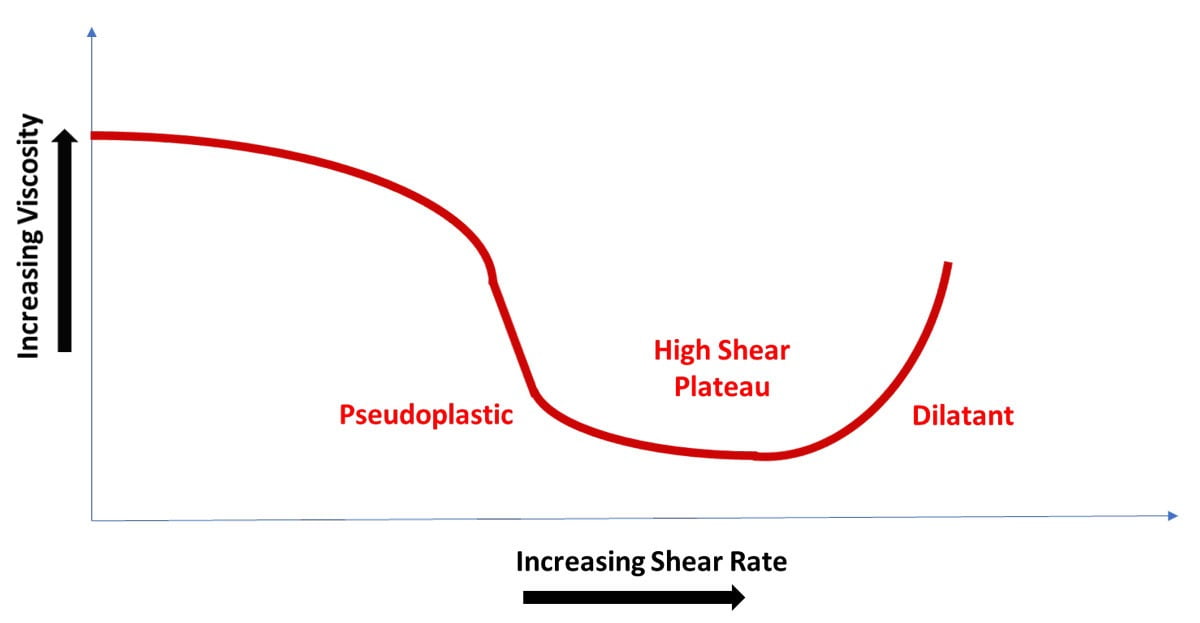
FIGURE 2 ǀ Viscosity profile of a coating.
In coatings, shear deformation is most commonly used and can be measured over different ranges. The exceptions are spray applications where extensional deformation determines the droplet size. Spatter from a roller is elongation viscosity and can be measured, but it is not common. Viscosity can and should be measured at different shear rates. While low, medium and high are subjective, low shear rate measurements are best to assess settling, sag and leveling. Medium shear rate measurements for coatings are good for dip coating, flow coating and brush application. High-shear measurements are best for brush or roller applications. These processes occur at different shear rates depending on the system being formulated. Historically, single-point viscosity measurements were used, but the emergence of affordable rheometers that can measure viscosity over a range of shear rates has improved rheology optimization in coatings. Single-point viscosity measurements pose a huge potential for error when additives with varying shear rate dependence are used. Two coatings may have the same viscosity at a single point, i.e. shear rate, but if they differ in having more or less pseudoplastic behavior, application differences can become apparent.
Viscosity cups (Zahn, Ford, DIN) are used for low- to medium-shear measurements. Viscosity cups are containers with a hole in the bottom, and measurements are performed by timing how long it takes the cup to empty. The cup shape and size are determined by the type, followed by a number for the size of the orifice. They are used for low-viscosity liquids such as conventional spray coatings or stains. Their use is limited by a high variation in results due to user variability, and they can only be accurately used for near-Newtonian liquids. Their low cost and ease of use make them very common. Some are designed to fit through the bung hole in a container, so removing a sample is not necessary.
Spindle and paddle viscometers (Brookfield, Stormer) are used for medium shear rate measurements. They have traditionally been used for medium- to high-viscosity coatings. Spindle viscometers cover a wider viscosity range than paddle viscometers, but in higher viscosity systems they can channel, leading to measurement error. For a spindle viscometer to accurately and repeatedly measure viscosity, the rotating spindle induces consistent laminar flow over the spindle. Therefore, we wait for the system to equilibrate before taking a measurement. If the viscosity is too high, especially in pseudoplastic liquids, new liquid may not reach the spindle fast enough to replace liquid displaced by laminar flow, i.e. laminar flow is interrupted. Consequently, a channel around the spindle is created that gives inconsistent or inaccurate readings. Paddle viscometers have offset paddles to minimize channeling and work best for medium- to high-viscosity coatings. These types of viscometers have traditionally been used for architectural coatings (paddle) and industrial coatings (spindle).
Rotational viscometers (cone and plate, parallel plate) can be used for any shear rate, but are generally used for high-shear measurements. They are best for determining how a coating will behave in brush, roller or spray application.
Ideally you want a coating with a high viscosity at low shear to prevent pigment settlement or syneresis, low viscosity during application to aid in flow and leveling, followed by a quick return to high viscosity when shear is removed to prevent sag and drips. Therefore, pseudoplastic behavior is desirable in coatings. The problem is the coating has time-dependent behavior. Thixotropy is when a pseudoplastic liquid is sheared and it does not return to the original viscosity. The more thixotropic a liquid, the greater the change in viscosity at low to no shear. This means that if a coating is thixotropic it can be unstable, and if it is sheared many times, it may have lower viscosity, which will affect settling and application (see Figure 1 for pseudoplastic and thixotropic behavior). Pseudoplastic and thixotropic are often used interchangeably but should not be. Figure 3 highlights the shear rate for common coatings applications.

Photo courtesy of Indorama Ventures.
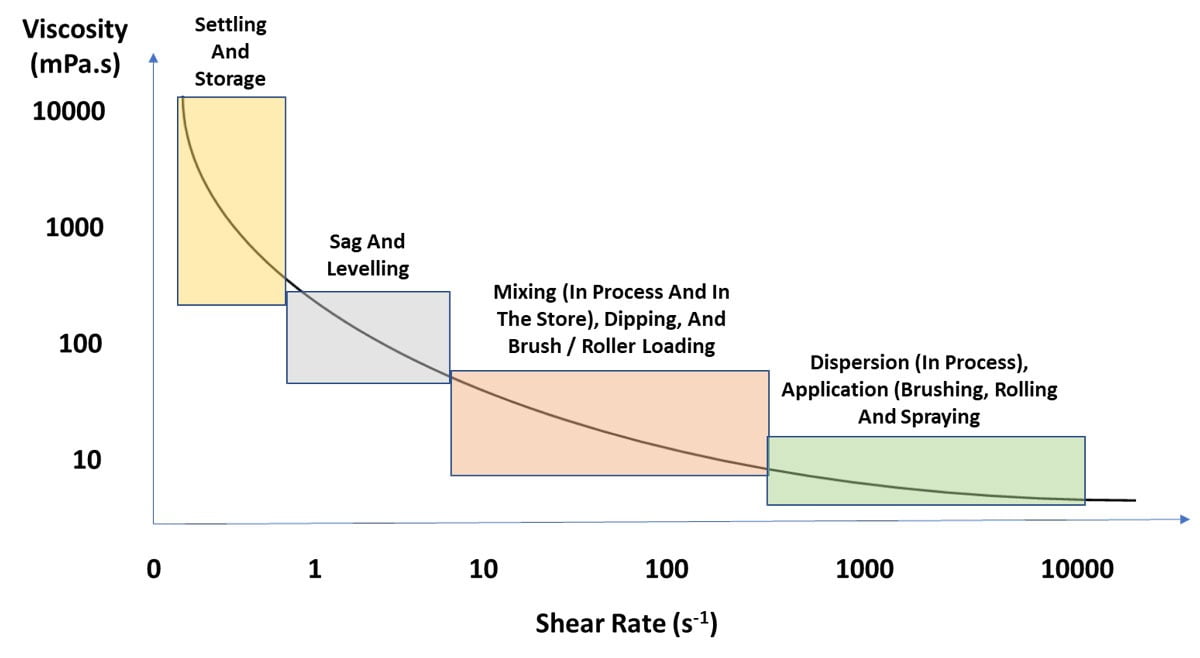
FIGURE 3 ǀ Viscosity and shear rate ranges for common coatings applications.
Suspension Agents
Anti-settling agents (commonly called settling agents) or suspension agents are used to prevent pigments or other solids from settling out in a coating. Stokes law shows that viscosity is only one of the factors that affect settling (particle size, density, shape and frictional forces also influence settling rate). The purpose of an anti-settling agent is to prevent the solids from falling out of solution. They can also help prevent hard-pack settling, allowing the particles to be easily re-incorporated.
Stokes Law
Fd = 6πµRνs
Fd = Frictional force or Stokes drag
µ = Dynamic viscosity (newton seconds/meter2)
R = Radius of the particle
νs = Settling viscosity of the particle = 2x(particle density‑liquid density)x(gravity)x(radius2).
9 µ
It is important to know the density of the liquid and the particles. Particles may rise instead of settle if the density of the particle is less than the liquid. Hollow glass spheres, polymer pigments, waxes and others may have a lower density than the liquid phase in the coating.
The last effect of a rheology modifier or settling agent is to prevent syneresis or liquid separation in a coating. This is the stratification of different components in the coating based on their density. Syneresis or liquid separation can be anything from a light layer of solvent (remember water is a solvent), to resin in the bottom of a container. In extreme cases of syneresis, the resin separates from the water in the coating and can go through the coalescence process and form a solid mass.
Rheology, viscosity, anti-settling and syneresis modifiers are extremely important in a coating formulation, and can have a more pronounced effect than most other additives. It is important to remember that rheology modifiers are designed to increase the low shear or the overall Newtonian viscosity.
Rheology Modifier Types
Alkali Responsive Thickeners
Alkali-Swellable Emulsions (ASE) and Hydrophobically Modified Alkali-Swellable Emulsions (HASE) are shear-thinning emulsions used for anti-sag and pigment suspension in water-based systems. They also can affect the application viscosity of the coating, changing sag and levelling properties. HASE thickeners are hydrophobically modified versions of ASE, through the addition of hydrophobic functional groups, and tend to increase viscosity greater than ASE for the same level used due to the combination of their alkali-swellable and associative nature. Both are low-viscosity liquids that thicken up and greatly increase viscosity when the pH is raised above a threshold. ASE are non-associative, while HASE tend to be associative because of the hydrophobic functional group. The disadvantage of alkali-swellable thickeners is that if the pH drifts outside the ideal range, viscosity will drop and performance will suffer.
Nonionic Synthetic Thickeners
Hydrophobically modified Ethoxylated URethane (HEUR) and Hydrophobically Modified PolyEther (HMPE) work by having the hydrophobic region associate with the resin, setting up a 3D network of associations. Because of the non-ionic nature of these additives, they will have less effect on moisture vapor transmission through the film than ASE and HASE emulsions. They operate by associating (aggregating) together in the water phase and associating with resins or other hydrophobic ingredients. Other additives or solvents can increase or decrease the associative nature of the associative thickener, leading to a change in efficiency. Their performance is also much more independent of pH than the alkali-swellable emulsions. Figure 4 illustrates how they work.
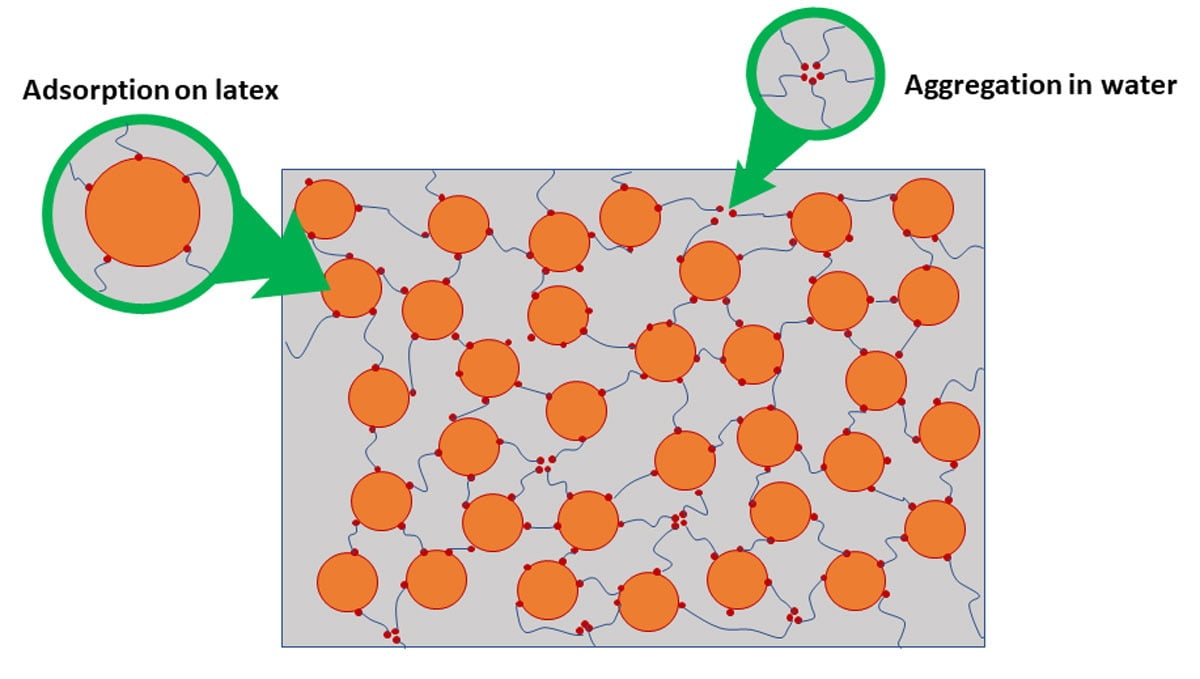
FIGURE 4 ǀ How associative thickeners work.
Cellulosic Thickeners
Cellulosic thickeners are insoluble in water and have an inflexible polymer backbone that works by thickening the water. They impart a low-shear viscosity and have little effect on high-shear viscosity. Cellulosic thickeners also tend to be more pseudoplastic than is ideal. They are generally Hydroxy Ethyl Cellulose (HEC) or modified HEC, and raise viscosity through hydrogen bonding with water. They offer reasonable properties for some paints as a single thickener, and provide good resistance to sagging, settling and syneresis. However, cellulosic thickeners increase the probability of flocculation, lower gloss, reduce washability, promote roller spatter, are prone to microbial attack, and only work in water-based systems. There is also Hydrophobically Modified Hydroxy Ethyl Cellulose (HM-HEC), which through the hydrophobic modification will allow associative thickening as well as the standard HEC thickening mechanism.
Inorganic Thickeners (Clay and Silica)
Clays are naturally occurring hydrated aluminum silicate and are rarely used as a sole thickener. Kaolin clay is the most commonly used clay. Other clays found in coatings include attapulgite and bentonite. Clays can impart thixotropy, prevent syneresis and improve sag resistance. However, clays will decrease gloss. Kaolin and attapulgite clays are non-swelling, while bentonite is considered swelling. Due to the high aspect ratio of clays combined with charge stabilization (polar nature with edges and centers having differing charges) you set up a house of cards-type stabilization. Clays work in water-based, solvent-based and 100%-solids systems, however some polar nature is needed to exfoliate the particles and provide the stabilization.
Silicas form a loose, lattice-like network by hydrogen bonding between particles. The network is stable at rest, but is easily disrupted by shear forces, and rebuilds when the shear is removed. Silica can be hydrophilically and hydrophobically modified to work in all coatings systems. They impart thixotropy and provide sag and settling resistance. They cannot be used as a sole thickener, can lower gloss and may be pH sensitive. Table 1 shows the differences between clays and silicas.
TABLE 1 ǀ Comparison of organoclays and fumed silicas.
There are many other rheological modifier types, including modified castor oils, polyamide, etc., but due to the brevity of this article, only the main types were considered.
Generally, you will not formulate with only one thickener, as combining them improves the properties of the final coating. Common combinations are clay and cellulosic for inexpensive water-based coatings; and associative, clay and cellulosic for higher performing water-based coatings. For solvent-based and 100%-solids systems, a combination of clay and silica works best. Table 2 compares some common thickeners.
TABLE 2 ǀ Comparison of major rheological modifiers.
Conclusion
In conclusion, understanding additive use and choosing the proper combination of additives will have a drastic effect on both a coating’s performance and cost. Understanding rheological modifiers will help increase the stability of the system as well as maximize the application performance of the coating. The next installment of this series will cover foam-control agents.

Mike Praw
Author’s Bio:
Born and raised in Montreal, Mike now lives in the Houston area. He has 34 years of coatings formulation experience, 18 years with coatings companies and 16 years with raw material suppliers. He is currently Senior Applications Scientist – Paints, Coatings and Inks, for Indorama Ventures: Integrated Oxides and Derivatives. He has degrees in Analytical Chemistry and Environmental Sciences, as well as a MBA. Mike is the Past President for The Detroit Society for Coatings Technology and The Piedmont Society for Coatings Technology, and served on the board of the Chicago Society for Coatings Technology. Mike is a Canadian Armed Forces veteran, having served 15 years in the Canadian Infantry.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers and directors.
