Ready to proof -- Clare 10/5/22
CB proofed 10/11 - left comment for KJ
KJ proofed 10/11. Clare, see the note by the figure. We need to add FIGURE 1 above it, with the caption indicated in the note. Thanks.
Revised on 10/11
KJ: clean from my end
DID YOU KNOW?

How are Surfactants Distributed within Latex?
Video credit: ilyast / Creatas V1ideo, via Getty Images
As we all know, the surfactants we add to synthetic latexes have allowed us to create wonderful nanoparticles and at the same time provide colloidal stability (against salts, freezing/thawing, and mechanical shear) to the dispersions. But they also can cause foaming problems when mixing and transporting the latexes, and remain in the final products causing potential water absorption problems in films and coatings. However, on a commercial scale, they are necessary.
Perhaps it goes without saying, but it should be obvious to us that the surfactants will be distributed between the water phase and the particle surface, and the question then arises as to what that distribution looks like. But first, are the surfactants also inside the polymer particles? For anionic or cationic surfactants, the answer is a resounding no. In fact, common anionic surfactants like sodium dodecyl sulfate (SDS, or SLS) are not even soluble in the monomers we use to make the polymer. Non-ionic surfactants present a different situation as some are soluble in the monomers we choose, but not usually soluble in the final polymers. This results in the potential condition that when non-ionics are added to a batch polymerization (lots of monomer in the particles), some of that surfactant might become absorbed inside the particle in the early stages of the polymerization, and perhaps get trapped within the particles in the latter part of the polymerization.
Certainly, there are some complexities here. But for the anionic (or cationic) surfactants, the situation is simpler. Here we only have to ask how the surfactant distributes between the water and the particle surface. It is clear that when the surfactant concentration in the water phase is at its critical micelle concentration (CMC), surfactants on the particle surface must be packed to maximum spatial density (i.e. any more surfactant added to the latex would just form more micelles). This CMC condition allows us to measure how much of the total surfactant is on the surface of the particles at maximum packing. That number is commonly designated as AS, with units of Å2 of particle surface area per surfactant molecule. This value is known to vary with the type of polymer at the surface of the particle – the more polar the polymer, the higher the AS value (meaning less densely packed).
Now consider the situation in the figure below where we have imagined a composite particle with two polymers of differing polarities, each presenting its own surface to the water phase. One can see here that at a fixed level in the latex, the surfactant is distributed between the water and both polymer surfaces at the same time, all conditions being at some thermodynamic equilibrium.
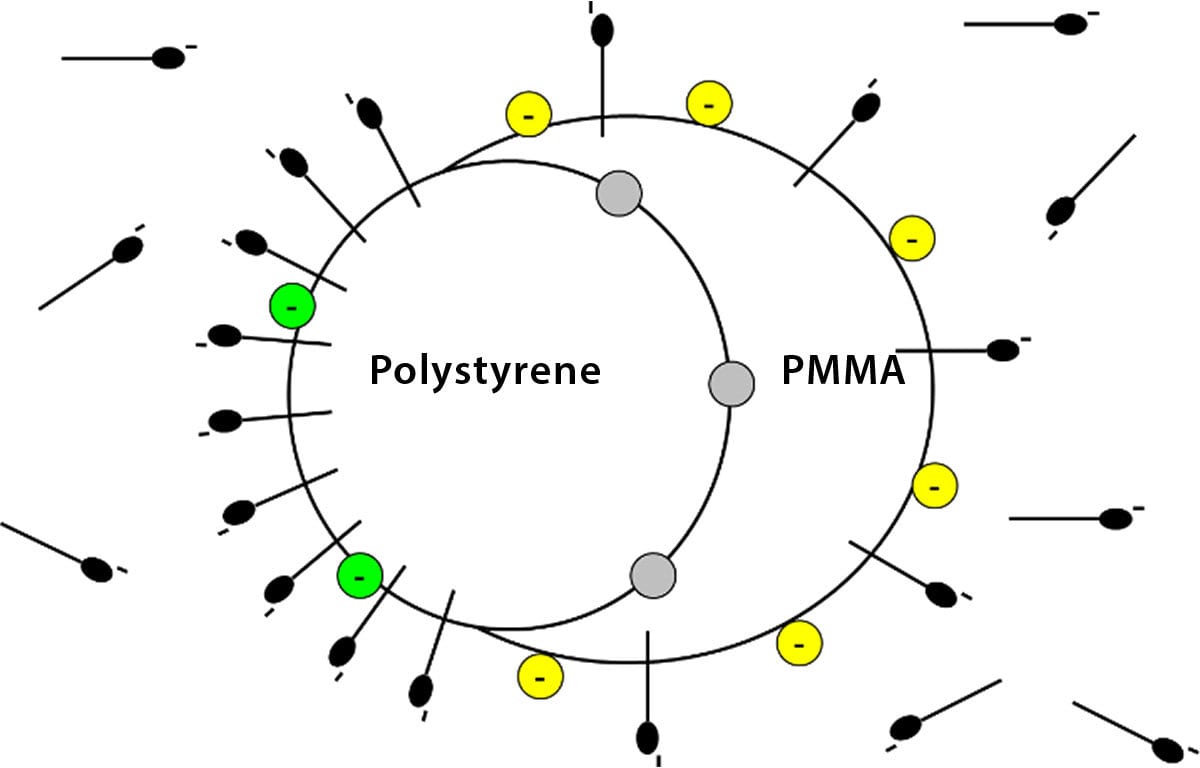
FIGURE 1 ǀ A composite particle with two polymers of differing polarities.
This interesting situation, at all levels of surfactant loading, is a topic we treat in detail in several of our STEPn workshops. We have also published some results for physical blends of different latexes [Langmuir, 15, 3250 (1999)]. As always, we invite your questions and comments via our website, www.epced.com.

The “Did You Know….?” series is a bi-monthly note from Emulsion Polymers Consulting and Education (EPCEd) that is intended to present simple questions about topics that are important to those working in the emulsion polymers area. Short and concise answers to those questions are presented to educate readers and to elicit comments and further discussion. Some readers will already know the answers and be familiar with the topic, while others, especially those newer to the field, will benefit from the answers and discussion. Experienced practitioners may also find new insights in the discussion. Paint & Coatings Industry magazine has partnered with EPCEd to share the “Did You Know” notes with our readers throughout the year.