Ready to proof -- Niki - 10-16-23 - edited 10/12
KJ proofed on 10/12. One note for Niki below (regarding Figures 1-4)
Ready for author
Video: icetraystanislav, Creatas Video+, via Getty Images
Surface Preparation of Steel Surfaces and Passivation by Catechol Polymers
By Charles White, Naval Surface Warfare Center, Bethesda, MD; Nevin Naren and Jonathan Wilker, Department of Chemistry and School of Materials Engineering, Purdue University, West Lafayette, IN; and Jordan Ravenelle, Elzly Technology Corp., Reston, VA
Complex geometries on metal surfaces reduce coating performance and provide sites for corrosion, coating delamination, and coating abrasion. An effective corrosion preventive pretreatment and adhesive tie coat is desired for improving coating performance on U.S. Marine Corps (USMC) ground vehicles to enhance the adhesion of USMC chemical agent-resistant coatings (CARC) primers and topcoats. The Naval Surface Warfare Center, Carderock Division (NSWC-CD) Corrosion and Coatings Engineering Branch (Code 613) performed corrosion evaluation and coating compatibility testing on panels prepared with poly(styrene-co-catechol)-based adhesive pretreatments for improved coating adhesion and improved corrosion resistance for U.S. Marine Corps ground vehicles. Here, NSWC-CD presents the results of the ongoing tie coat prototype development for improved adhesion of CARC to steel.
Background
Coatings and coating systems used on the exterior of USMC and U.S. Army ground vehicles are prone to weather and water exposure, and must be able to protect the metal substrate from corrosion, and maintain a high level of coating adhesion and performance. Ground vehicles are composed of diverse materials/substrates, including steel, stainless steel, composites, and aluminum. This makes them difficult to properly coat and maintain performance requirements and aesthetic appearance while maintaining operational availability for the uniformed services. USMC ground vehicles are required to be coated with a qualified chemical agent-resistant coating (CARC) in a standard camouflage pattern. CARC is applied in a system with an anti-corrosive primer and a camouflage topcoat in various colors. The CARC paint system is engineered to be protectant against radioactive, biological, and chemical contamination.
Corrosion prevention along complex geometries, over flexible materials, and along sharp edges extant on ground vehicles can provide unique performance challenges, particularly for high-performance demands often experienced by USMC ground vehicles in the form of impact and abrasion. Loss of CARC adhesion to different metallic substrates, such as 6061 and 5083 aluminum (aluminum alloys that have other elements added to pure aluminum to enhance its properties), stainless steels, fasteners, and carbon steels has been documented across USMC ground vehicles (Figures 1-2). The high additive and filler content of CARC formulations can contribute to loss of adhesion to different metals due to chemistry and surface preparation, and operational conditions of the ground vehicle (Figures 1-3). Loss of coating adhesion leads to frequent corrosion and repair, lowering the operational availability of USMC ground vehicles, while adding unnecessary cost and maintenance burden. The use of conversion coatings has shown improved weatherability, abrasion resistance, and adhesion of the CARC to the aluminum roll-up door panel (Figure 4).

FIGURE 1 ǀ Coating loss over aluminum on USMC ground vehicles.

FIGURE 2 ǀ Loss of CARC adhesive to USMC ground vehicle.

FIGURE 3 ǀ Ground vehicles undergoing repair and/or storage after use.

FIGURE 4 ǀ Improved adhesion of CARC through use of a conversion coating, left: conversion coated, right: SSPC-SP 1 only preparation.1
Developed as a bio-inspired adhesive, the poly(styrene-co-catechol) resins (Figure 5) can stick to a variety of substrates in both dry and wet conditions. The hydrophobic styrene backbone helps to drive away water from the metallic surface to allow for catechol binding to the surface. This surface binding of the exposed catechol can occupy potential corrosion sites, and the mix of exposed catechol and styrene backbone provides a unique surface for primer and topcoat binding. The unique redox chemical characteristics of catechol allows for the incorporation of crosslinkers into the formulation. An added crosslinker could serve as both a reagent to improve corrosion resistance (i.e., a passivating reagent such as zinc), and a reagent to link polymer chains toughening the coating or adhesive.
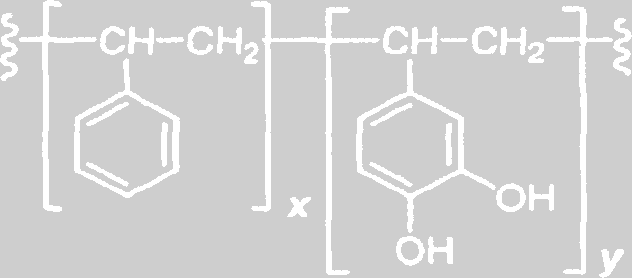
FIGURE 5 ǀ Poly(styrene-co-catechol).
Unlike most two-part epoxy systems or chemical pretreatments that are typically chemically cured, the poly(styrene-co-catechol) formulation dries via solvent evaporation. The polymer is formulated and cleaned prior to incorporation into the coating formula. This processing approach converts the hazardous styrene monomer into the polymer, and any residual monomer is removed during the later steps. The final polymer can then be handled as common waste without the hazardous waste handling required for chromium-containing coatings. Inclusion of zinc phosphate or aluminum triphosphate will promote the creation of an inhibited, or passivated, metal layer on the applied substrate. Typically, zinc phosphate in pretreatments must be applied over 80% to create a passivated layer in liquid conversion coatings. However, incorporation of zinc phosphate in a hydrophobic polymer may reduce the required zinc content for effective passivation. Zinc concentration inversely correlates to important coating performance properties like adhesion and flexibility, i.e., reducing the concentration of zinc in the coating allows for greater coating physical performance. Alternatively, soluble zinc compounds could be dissolved in the polymer solution with a phosphate counter ion. During cure, the zinc phosphate will precipitate evenly into the pretreatment. This strategy could facilitate the use of less zinc with similar performance as the currently available high zinc coatings. The use of a pretreatment that continuously inhibits corrosion prior to the application of a coating system will prevent both corrosion and coating failure while promoting topcoat adhesion to the treated surface.
Experimental Procedure
Coatings were evaluated in two concurrent efforts: electrochemical impedance spectroscopy (EIS) characterization with CARC compatibility of the poly(styrene-co-catechol) resin, and EIS characterization with CARC compatibility of the zinc-laden poly(styrene-co- catechol) resin. Two products (Products A and B) were developed using poly(styrene-co- catechol) resin at different molecular weights and solvents for evaluation (Table 1). EIS analysis was performed to measure corrosion inhibition for up to 12 months compared to a blasted steel panel and qualified CARC system. CARC compatibility was determined as per MIL-DTL- 53022E and MIL-DTL-53039E requirements and atmospheric exposure in Fort Lauderdale,FL.2-3
TABLE 1 ǀ Product descriptions.
Panel Preparation
Pre-blasted, hot rolled steel panels (2-3 mils surface profile) were procured from KTA Tator (Pittsburgh, PA). Panels (either 6” x 12” x ⅛” or 4” x 6” x ⅛”) were cleaned in accordance with SSPC-SP 1 (isopropanol) and dried at ambient laboratory conditions (70 ± 5 °F, > 50% relative humidity).1 The catechol products were applied by nylon/polyester brush to completely cover the panel surface and dried at ambient laboratory conditions (Figure 6). The drying time was recorded for each product.

FIGURE 6 ǀ Product A applied to prepared steel panels.
The paint schedule is provided in Figure 7. Once the tie coats had dried, a qualified anti-corrosive CARC primer (MIL-DTL-53022E) was applied in accordance with the manufacturer’s product data sheet and dried at ambient laboratory conditions.2 After 24 hours, a qualified green CARC topcoat (MIL-DTL-53039E) was applied (except for EIS panels) in accordance with the manufacturer’s product data sheet and allowed to dry at ambient laboratory conditions (Figure 8).3
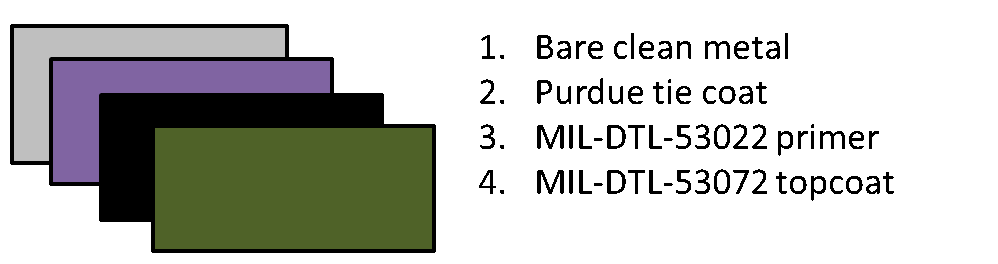
FIGURE 7 ǀ Paint schedule showing SSPC-SP 1 bare steel (gray), brush-applied poly(styrene-co-catechol) products (purple), qualified CARC anti-corrosive primer (black), and qualified CARC topcoat (green).

FIGURE 8 ǀ CARC topcoated panels.
Results
Two prototype products were developed for this evaluation (Table 1). Both products are brush applied poly(styrene-co-catechol). The products utilized diverse poly(styrene-co-catechol) resin molecular weights and solvents, and were formulated to different percent solids.
Initial characterization was performed through EIS. Three panels, i.e., a bare steel panel, a poly(styrene-co-catechol) Product A-coated panel, and a poly(styrene-co-catechol) Product A, and CARC primer-coated panel were installed in the experimental set up (Figure 9, Figure 10, and Table 2). An uncoated, flanged stainless steel fastener was placed in the center of each cell, isolated from the panel with a rubber grommet. The fastener and panel were externally connected by a wire. The isolated cell area was immersed in artificial seawater for six weeks. After two weeks, the first EIS scans were attempted. After the initial six weeks immersion, artificial seawater was emptied from the cell and the system was dried out for two weeks. The panels then entered a two weeks wet / two weeks dry cycle for the remainder of testing.
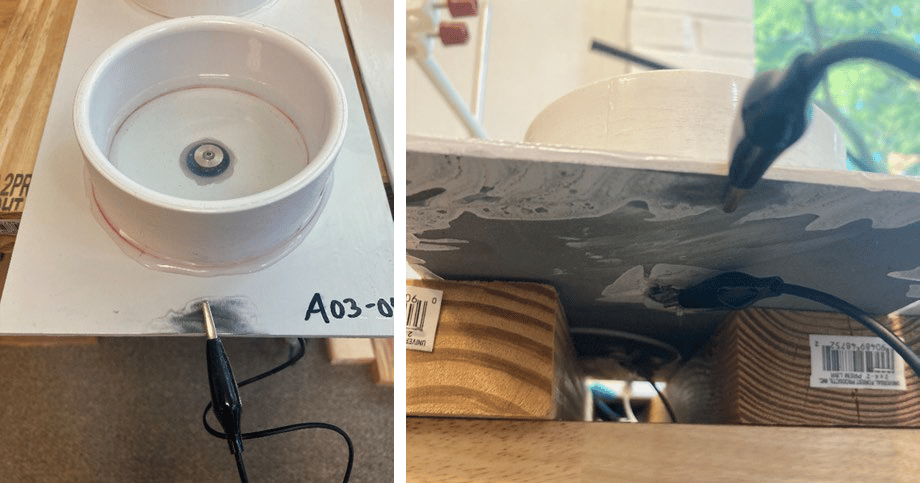
FIGURE 9 ǀ EIS test setup.
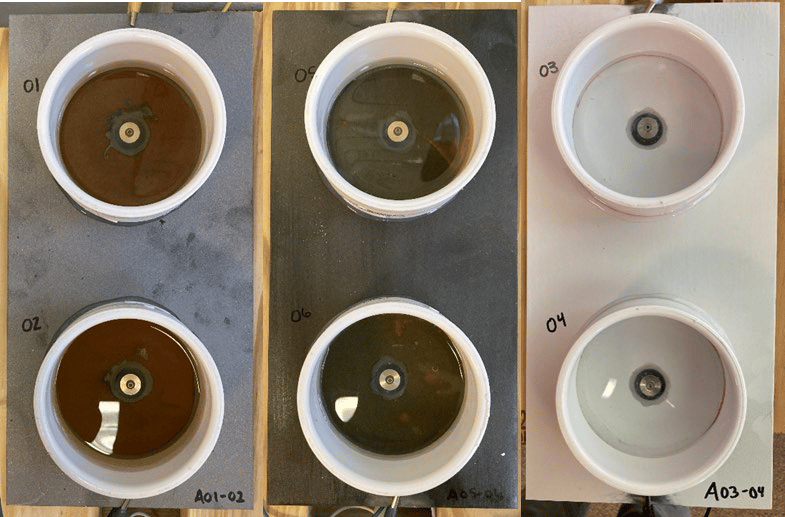
FIGURE 10 ǀ EIS test panels including (left) bare steel reference, (middle) Product A-coated steel, and (right) a qualified CARC primer.
TABLE 2 ǀ EIS panel surface preparation and coating system.
Drying time of the two poly(styrene-co-catechol) products were evaluated through ASTM D1640.4 Product A took 24 hours before the CARC primer could be applied, whereas Product B dried almost immediately. The product to be developed is intended for application and transition to the corrosion repair facilities (CRFs), which operate with a high throughput tempo. The success of the developed tie coat product performance requires that the products provide corrosion protection while not affecting the production rate of the CRFs. The dry time for product B was highly preferred. EIS and 24 hours water immersion were used to evaluate the corrosion protection. The results are presented in Figures 11-12.
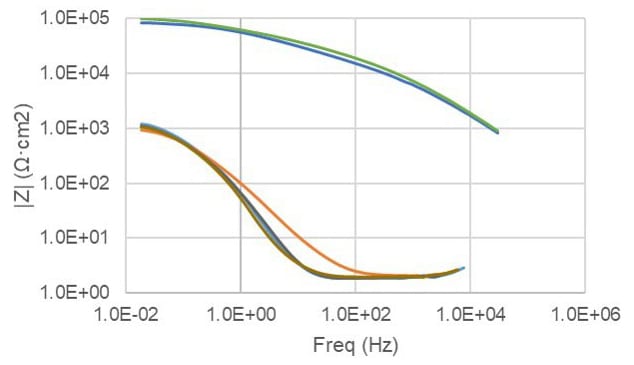
FIGURE 11 ǀ Bode magnitude plot results.
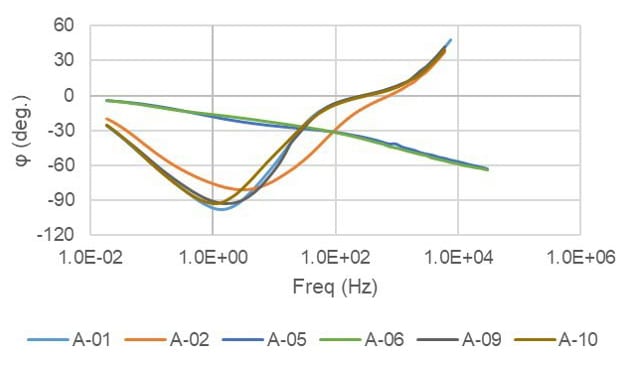
FIGURE 12 ǀ Bode phase angle plot results.
Product A (A-05/06) has greater low frequency impedance after 24 hours of immersion, indicating a level of barrier protection over and above bare steel (A-01/02) and Product B (A-09/10) (Figure 13).
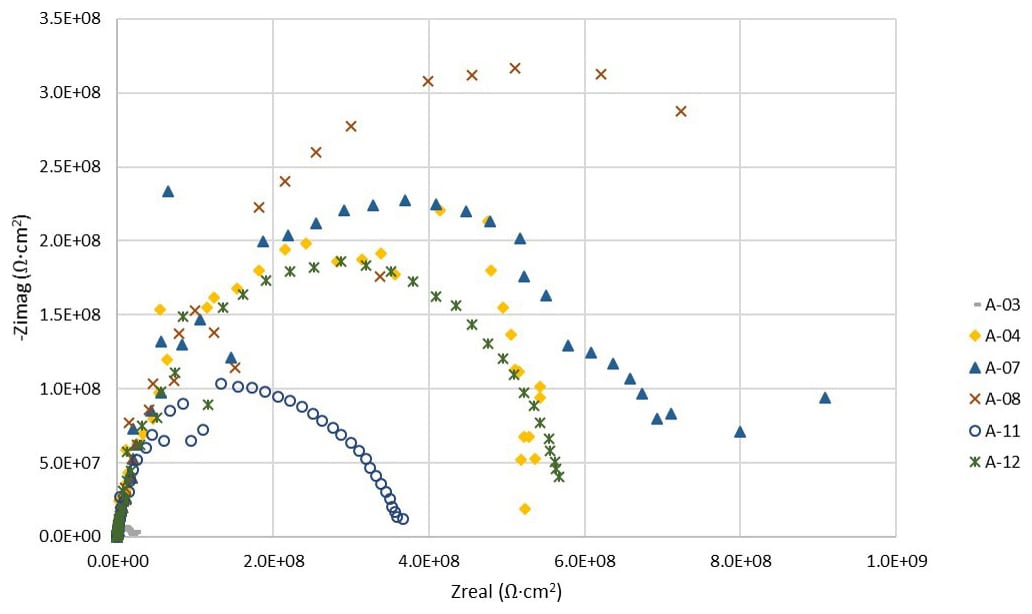
FIGURE 13 ǀ Impedances across all frequencies.
After three months of exposure to wet-dry cycling, the panels with the Product A adhesion promotor (A-07/08) have slightly higher impedances across all frequencies than those without any adhesion promoter (A-03/04) and those with the Product B film (A-11/12).
Note that A-03 had a holiday through which corrosion was visible from it after only a few days of immersion. This suggests that the Product A film may provide some benefit to corrosion resistance in addition to the CARC system. Six more months of wet-dry cycling and data collection are needed. The magnitude of current flow increased with exposure time for all panels. The current flow was approximately three orders of magnitude higher for panels without primer than with MIL-DTL-53022 primer.2 A-03 showed corrosion through the primer after a few days of exposure due to the presence of a holiday in the primer coat.
Water Immersion
Protection against corrosion was also evaluated through 24 hours water immersion of steel panels (Figure 14). Bare steel panels were immersed (left) in water. After 24 hours, bare steel panels have corroded (middle), whereas product A coated panels showed improved corrosion resistance. Product B showed no corrosion resistance compared to the bare steel. The assessment was qualitative, but demonstrated the corrosion resistance of product A.

FIGURE 14 ǀ Test panels immersed in water for 24 hours. Bare steel panels were immersed (left) in water. After 24 hours, bare steel panels have corroded (middle) where product A-coated panels showed improved corrosion resistance compared to bare steel.
The product A tie coat offers some corrosion protection, whereas the Product B tie coat does not. However, this corrosion protection is minor in comparison to the CARC coating system. The tie coat corrosion protection is developed for temporary corrosion protection until the CARC coating system can be applied. The real benefit of the tie coat will be if it improves long-term adhesion of the CARC system. Additional long-term testing (ongoing) is needed to fully characterize the impedance.
CARC Compatibility Testing
The compatibility between the poly(styrene-co-catechol) tie coat products, and the CARC system was evaluated through conformance to performance requirements in accordance with MIL-DTL-53039 and MIL-DTL-53022 CARC requirements (Table 3).2, 3 The evaluation is still ongoing, but early results indicate that the poly(styrene-co-catechol) resins do not impact the CARC performance and improve the adhesion of the CARC system to the metal substrates.
TABLE 3 ǀ Coatings evaluation to MIL specifications.
Conclusions and Ongoing Work
Naval Surface Warfare Center, Carderock Division (NSWC-CD) has an ongoing project funded through the Office of Naval Research (ONR) to develop a novel, corrosion-resistant poly(styrene-co-catechol) tie coat and adhesion promoter to improve CARC adhesion and performance to various composites, aluminum, and steel USMC ground vehicle surfaces. Laboratory testing has shown that low percent solids poly(styrene-co-catechol) coating can be effective as a temporary corrosion-resistant coating and shown to be compatible with the MIL-DTL-53039 and MIL-DTL-53022 CARC primer and topcoat. Testing is ongoing, but molecular weight, percent solids, and solvent selection are critical for performance. EIS and qualitative corrosion testing has shown that thin films of poly(styrene-co-catechol) can be highly effective at temporary corrosion control without detrimentally affecting the performance of the CARC coatings.
Ongoing work includes the optimization of the poly(styrene-co-catechol) formulations in percent solids, solvent selection, and molecular weight to provide temporary corrosion protection and improved CARC adhesion. In addition, corrosion inhibitors such as zinc dust and zinc phosphate are undergoing evaluation for the addition of corrosion inhibition to the tie coat. In addition, long-term atmospheric testing of the poly(styrene-co-catechol) formulations will be performed as well as in-service demonstrations on USMC ground vehicles for long term tracking and evaluation.
Acknowledgments
NSWCCD would like to thank the funding sponsor, the Office of Naval Research (ONR), ONR Project Manager, Dr. Kristy Hentchel, ONR program officer for bioengineering and biomanufacturing, and our collaborators and performers at Elzly Technology (Reston, VA), NSWCCD engineer, Dr. Lee Huntington, and Dr. Jonathan Wilker at Purdue University (West LaFayette, IN), and Mussel Polymers, Inc. (Bethlehem, PA).
For more information, email charles.j.white153.civ@us.navy.mil.
