Ready to proof -- CLJ 08/09/21
KJ sent corrections on 8/10
CLJ revised 8/11
KJ sent one more correction on 8/12
CLJ revised 8/12
Ready for author
Photo courtesy of Chemical Processing Services Ltd.
Poly-Mannich Rapid-Cure Epoxy Curing Agents
By Paul H. Jones FRSC, Managing Director, Chemical Processing Services Ltd.
Curing agents for epoxy resins come in many guises. Numerous amines are modified such that the derivatives have reduced hazard, enhanced processability, and improved cured performance and service life. One favored modification is the generation of a Mannich base,1 which enhances processing and performance characteristics but with the introduction of some hazardous feedstock, which if left unreacted, creates a health issue.
Background
We are very conscious of pursuing sustainable products, and have developed a variety of Mannich base epoxy curing agents utilizing sustainable feedstocks. Our Furalkamine2 curing agents are examples of pentosane biomass-derived biopolymers, as are the phenalkamines we offer. There are some instances when the characteristics of petrochemical grades cannot be entirely achieved without the use of synthetic materials. When this is the case, the objective becomes eliminating Substances of Very High Concern (SVHC) and improving the safety rating. In order to do this, newly formulated polymeric Mannich base grades have been developed and released. These new products differ from some of the conventional grades in that they have higher molecular weight than the counterparts derived from monomeric phenols, and they are all compliant with Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations.
Mannich Bases
The broad range of curing agents available for crosslinking epoxy resins make them some of the most versatile thermosetting materials available, and a preferred basis of systems for many applications. The addition reaction that allows the formation of a stable three-dimensional network that offers high resilience, no chemical byproducts, low shrinkage and outstanding adhesion provides the perfect platform from which to formulate. The use of amine derivatives is commonplace, although there are phenolic, anhydride, amino and other functional materials that can be employed to react with the epoxy functional material. While basic amines can be utilized, they are invariably modified to enhance safety, processing and performance. One such modification is the Mannich base, which can convey outstanding performance, especially in ambient-cured systems.
Conventional Mannich bases are condensation products that have historically been derived from phenol and substituted phenols, with substitution being mainly in the para or occasionally the meta position. Examples of the phenols generally employed in the manufacture of conventional Mannich bases are detailed in Figure 1.
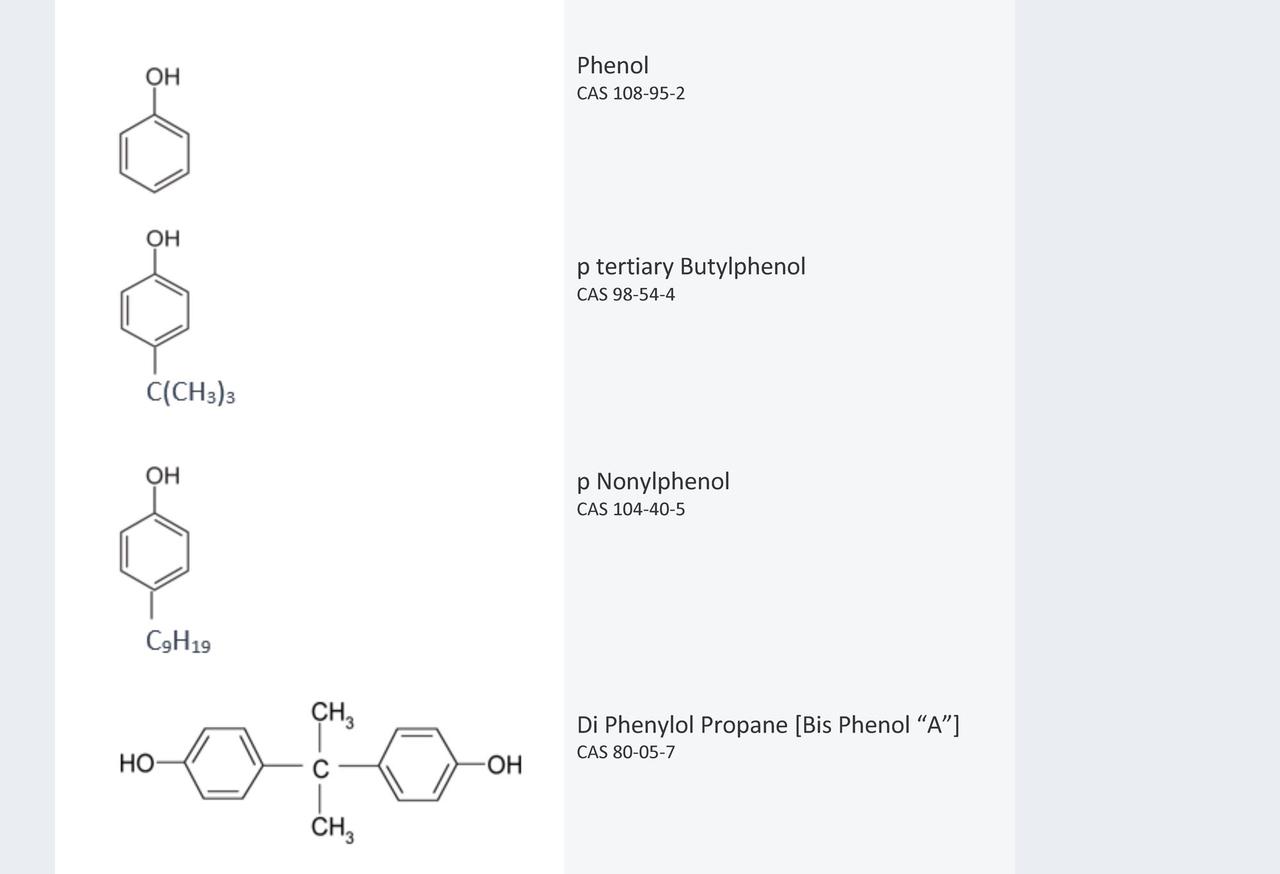
FIGURE 1 ǀ Typical phenols.
These phenols may be reacted with an aldehyde, almost exclusively formaldehyde, and a wide range of amines to generate the Mannich bases employed in the curing of epoxy resins. The selected phenol and amine obviously influence the resultant physical properties, Active Hydrogen Equivalent Weight (AHEW) and subsequent stoichiometry. These factors will influence the reactivity and the resultant cured properties, but of course these are also a function of additives and the epoxy component.
In general terms, irrespective of the phenol and amine selected, the aromatic nature of the phenol enhances compatibility with conventional epoxy resins, predominantly derived from bis phenol “A” and/or “F”; quite often a combination of both epoxy types to enhance the resistance of the epoxy component to crystallization.
Mannich bases enhance the compatibility of the amine with the epoxy resin constituent. There is a reduction in volatility and the hygroscopicity of the amine, the phenolic hydroxyl group accelerates the rate of cure and the combination of these characteristics in turn offers significant improvement to the surface appearance of a cured film. The reduced tendency for the amine to exude or migrate reduces or eliminates amine bloom. The improved cure rates, even at lower temperatures and under adverse conditions, make Mannich bases the curing agent of choice for some specific applications. Aside from the formulation of systems that allow all-season usage, a rapid return to service and cure in problematic environments, these products generally offer enhanced chemical resistance that can be further enhanced through selection of multi-functional epoxy grades (Figure 2).
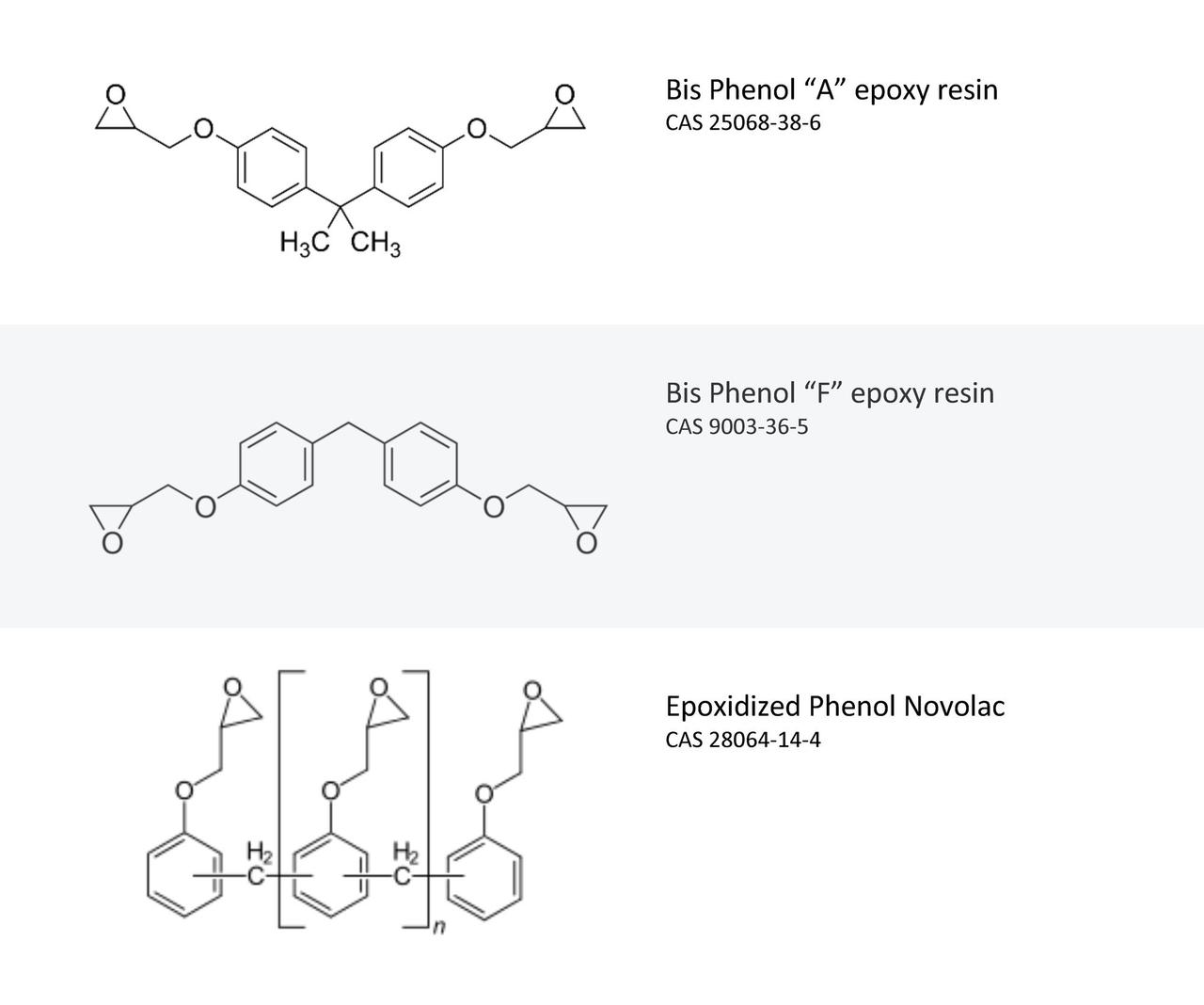
FIGURE 2 ǀ Typical epoxy resins.
As detailed previously, the conventional mannich base curing agents are derived from phenols and/or substituted phenols (Figure 3). While this reaction reduces the volatility of the amine, providing the enhanced and desirable properties associated with this product group, there are two negative elements to the modification.
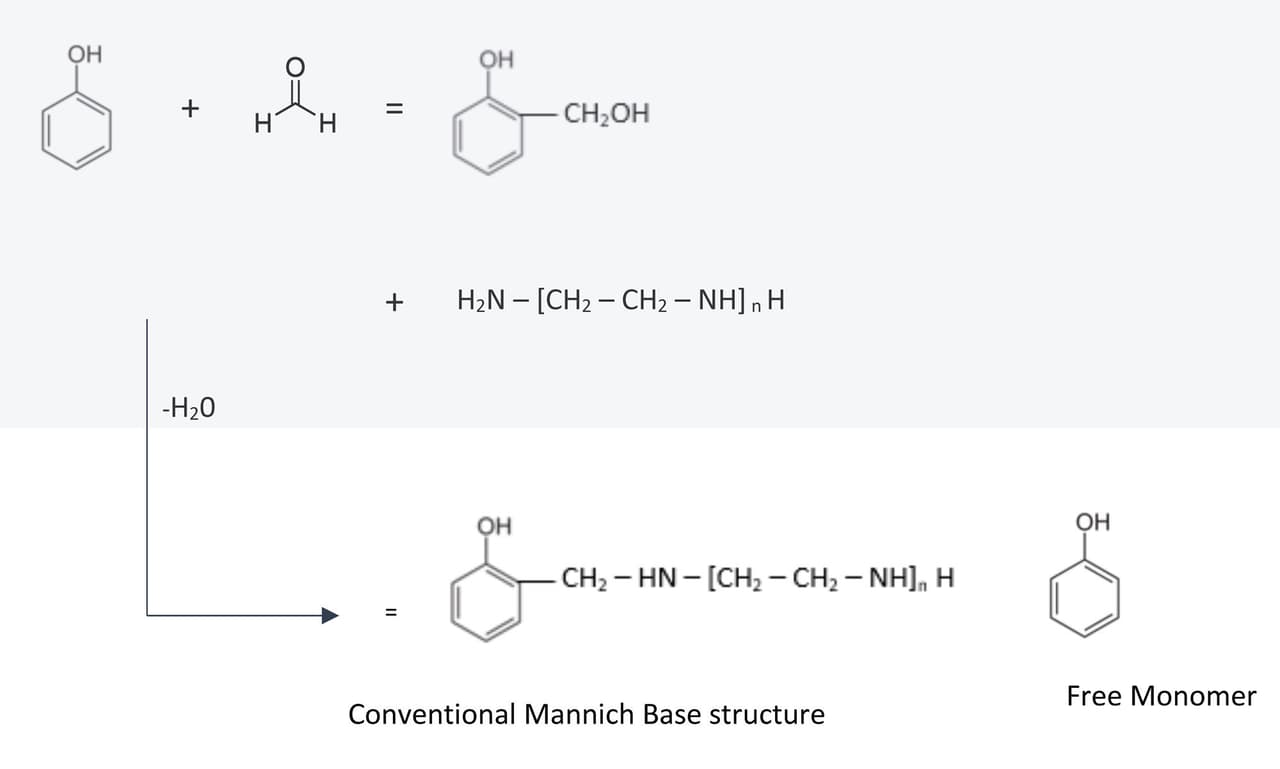
FIGURE 3 ǀ Conventional Mannich base process.
The first issue with the conventional Mannich base synthesis is the residual free monomer levels. Mannich bases can be formulated with a variety of ratios. There is generally an excess of amine and phenol, and there is invariably some residual phenol monomer(s) that lead to increased toxicity issues, as phenol itself is toxic, and many of the substituted phenols are of increasing concern.
Some tertiary amine mannich base accelerators, such as the industry-standard grade tertiary amine accelerator 2,4,6-Tris(dimethylaminomethyl)phenol1 is essentially formulated as a 1:3:3 ratio of phenol, formaldehyde and di methylamine. Of course, while targeting the tri-functional structure there are inevitably mono and di-functional derivatives, as well as some oligomeric structures.
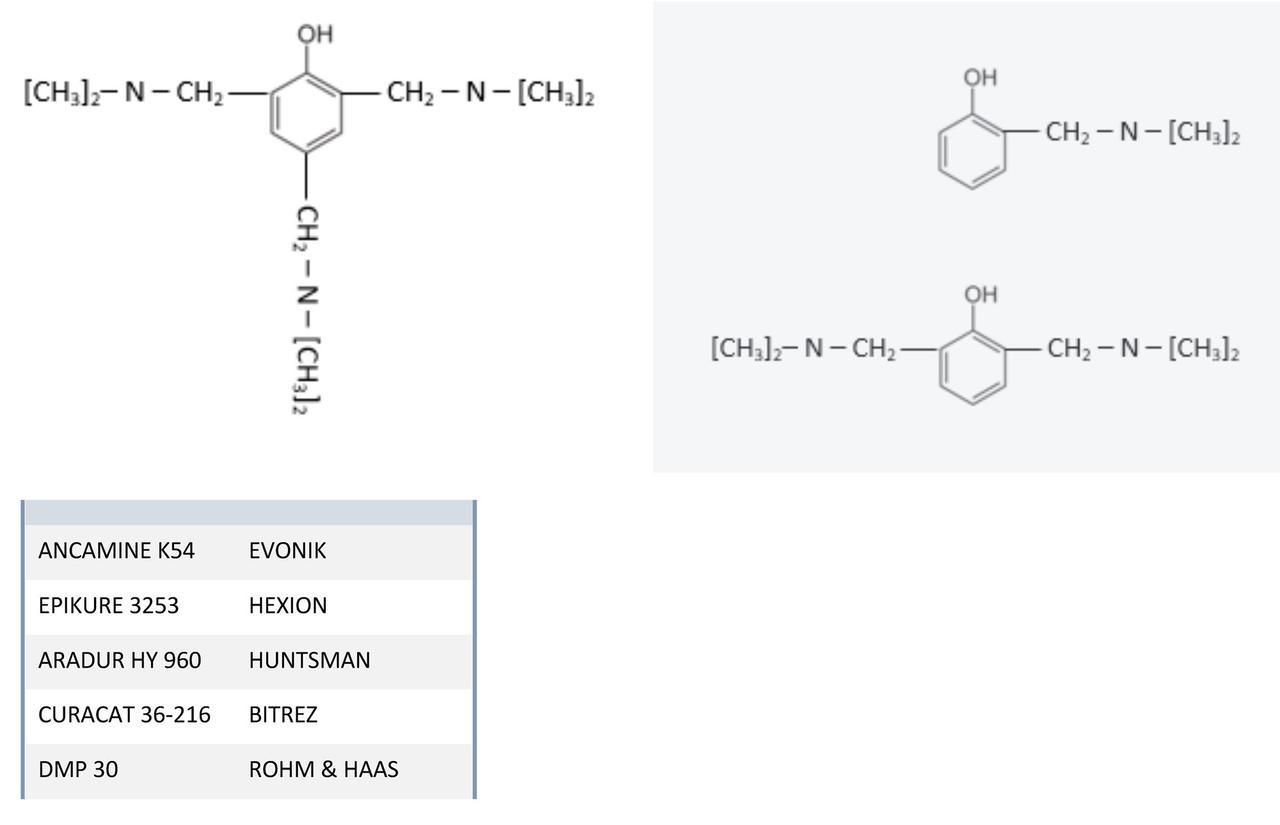
FIGURE 4 ǀ 2,4,6-Tris(dimethylaminomethyl)phenol.
While the above structure is processed such that the resultant material does not carry free phenol and its associated hazards, it does generate a low-molecular-weight material with very low polydispersity. The European Chemicals Agency (ECHA)3 guidance under REACH regulations defines a polymer as a material with a minimum of four covalently bound sequential units, with over 50% of the material being polymeric, and of the polymer, the molecular weight of over 50% of it must differ. Additional regional requirements may introduce the need for specific molecular weights, generally >1000 daltons.
To some extent the production of low free phenol Mannich bases can be overcome by the process of transamination. Combining the 2,4,6-Tris(dimethylaminomethyl)phenol with the preferred substituent, followed by heating and removal of the volatile low-molecular-weight amine, di-methylamine in this case, results in a functional Mannich base that has low free phenol. The process liberates problematic dimethylamine, and it is difficult to manage the degree and type of substitution. The resultant products also fail to meet the molecular weight requirements that classify them as exempt under the REACH guidance.
New Poly-Mannich Epoxy Curing Agents
Chemical Processing Services Ltd. has launched new PERMACURE poly-Mannich technology, which is a new category of epoxy curing agent. These new grades are polymeric Mannich bases that have the following chemical structure (Figure 5).
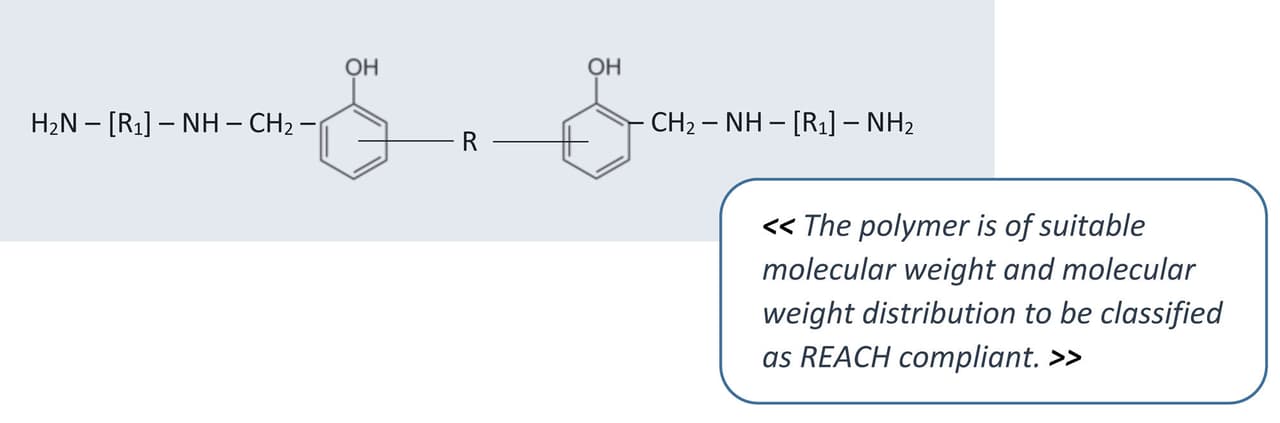
FIGURE 5 ǀ PERMACURE poly-Mannich structure.
The polymer is of suitable molecular weight and molecular weight distribution to be classified as REACH compliant and exempt as a polymer. There are several grades produced with different amines (R1) being the ethylene amine series, other aliphatic structures including poly-ether amines, aromatic, heterocyclic, araliphatic, or combinations, and so being analogous to the conventional types of Mannich bases currently available. The molecular weight of the feedstock is altered through the extension of R. These products also have <0.1% residual free phenol monomer or substituted free phenol monomer in the product avoiding the associated classification. Of course, they can be modified with such materials, although this is generally considered counter intuitive.
By way of example of the properties of the PERMACURE poly-Mannich grades, the conventional TETA-based phenol Mannich base CURAMINE 31-5374 is compared to an analogous TETA version of the new polymeric product PERMACURE H 5375 in Figure 6.
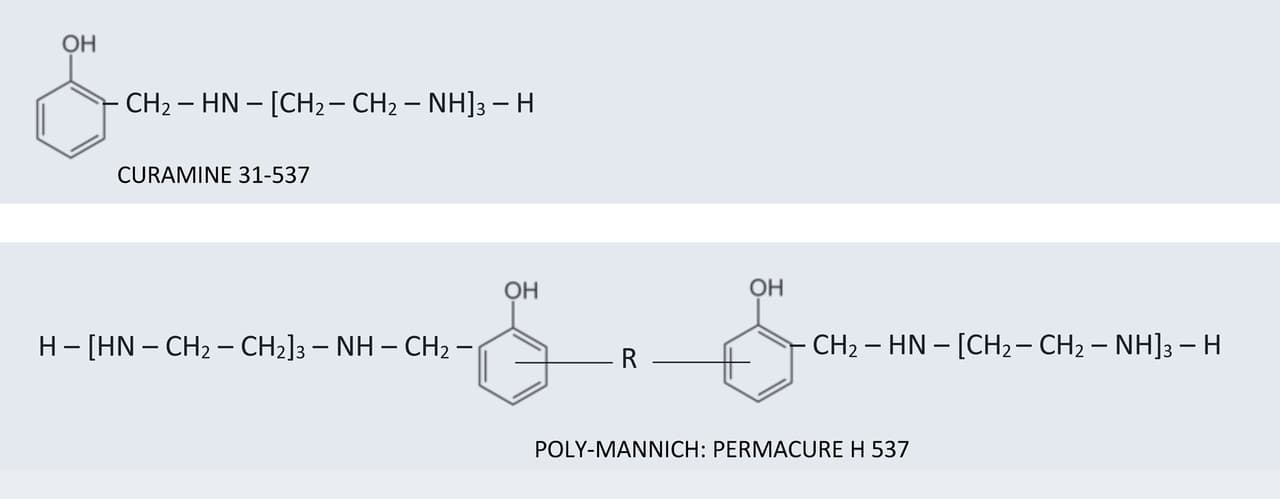
FIGURE 6 ǀ Comparative structures.
The following properties outline the differences between the product types.
Regulatory
The conventional Mannich base with residual free phenol carries both acute toxicity labeling and a chronic health hazard (Figure 7). These are not present in the new polymeric grades, although of course both grades are corrosive as a result of the amine from which they are derived. A full review is as follows.

Photo courtesy of Chemical Processing Services Ltd.
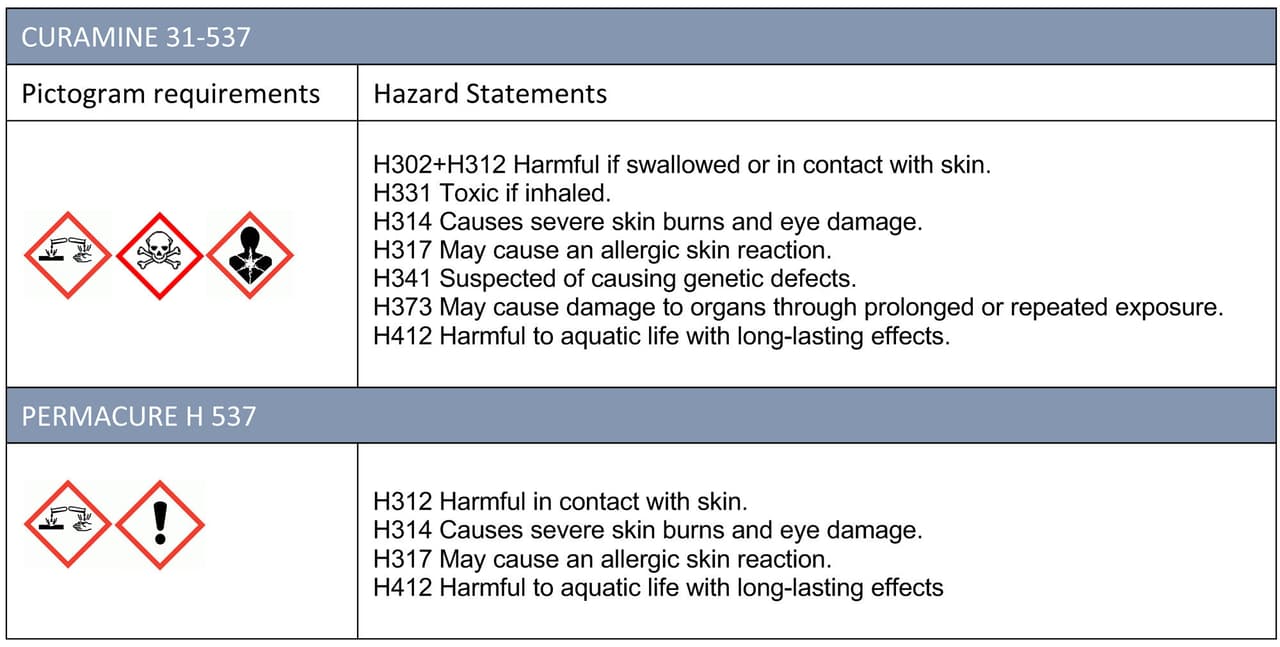
FIGURE 7 ǀ Pictogram and hazard statements.5
The removal of several of the hazard statements associated with free monomeric phenol and substituted phenols is now mandated by some organizations. The new grade can allow compliance with this requirement.
Property Comparison
The physical property comparison of the two TETA-based grades is detailed in Table 1.
TABLE 1 ǀ Comparative property values evaluated.
The property values are similar, but with some sacrifice with color and viscosity with the polymeric grade and a slight reduction in reactivity, as might be expected. Both products demonstrate good initial compatibility with several conventional epoxy resins, but relatively poor surface finish due to the residual free amine in both cases.
The simplistic TETA-based grades have similar performance, as depicted in Figure 8. However, if we continue to look we can see some of the further modifications offer enhanced cure speed, and cure response at low temperature and under adverse conditions. This is one of their key attributes and aids the formulation of systems offering a rapid return to service.
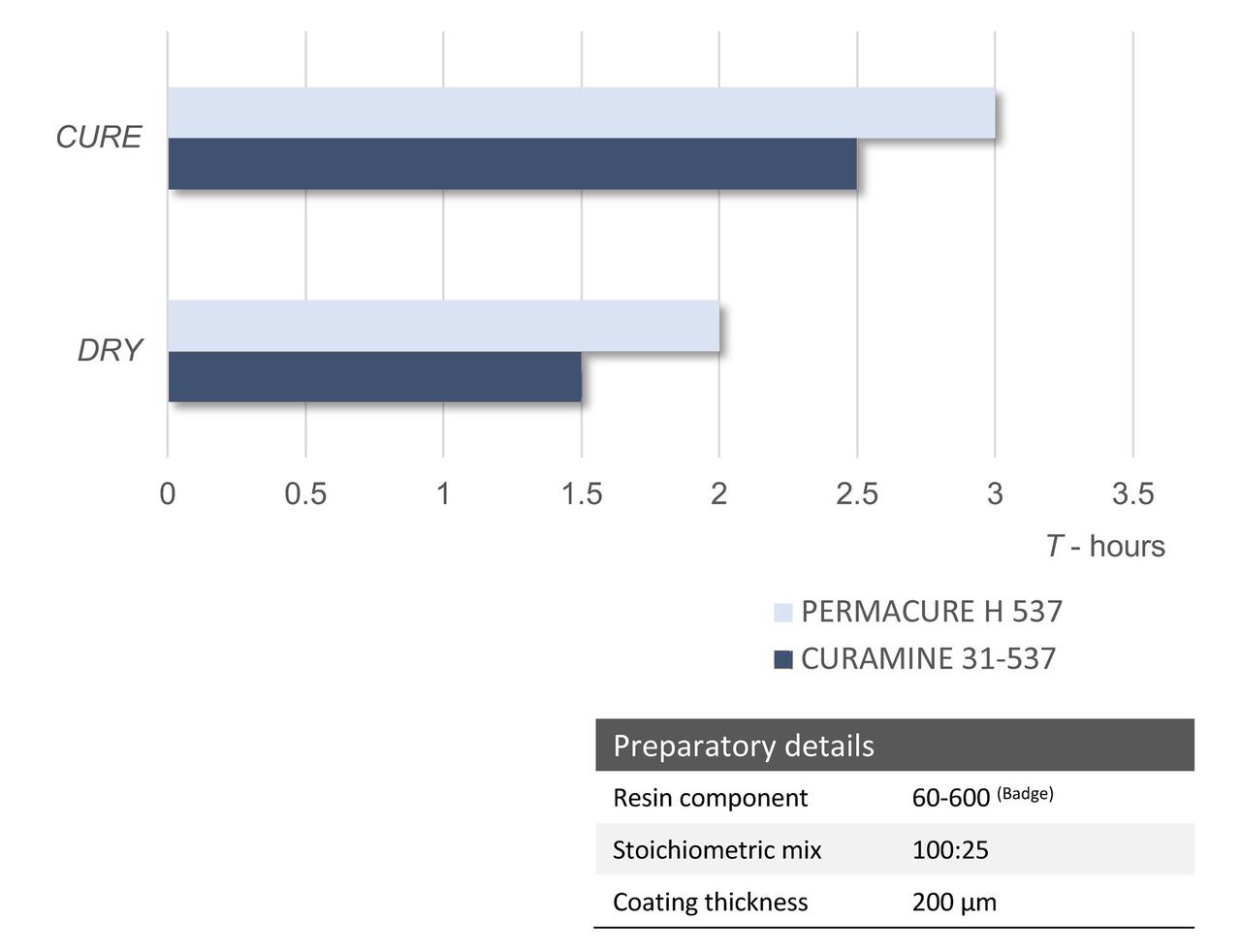
FIGURE 8 ǀ Dry times and cure times @ 25 °C.
The comparative dry and cure times are depicted above at a temperature of 25 °C. The cures at this temperature are relatively similar.
In order to fully utilize the benefits of this class of curing agent, not only has the backbone been altered to provide improvements in labeling, the functionality and structure of several grades have been further enhanced to improve handling and performance. An example of this is another of the new poly-Mannich grades, PERMACURE H531, which exhibits a much faster cure response, even at temperatures as low as 5 °C.
PERMACURE H531 is one of a series of these specially formulated grades characterized by low-moderate viscosity and extremely effective low temperature reactivity. This grade is one of the new polymeric Mannich base epoxy hardener series. This grade has an AHEW of 114 and mix ratio with a standard epoxy resin with EEW of 190 of 60 PHR. Thin-film reactivity is depicted below, demonstrating the excellent cure response. Cured films are clear, smooth and glossy at both 25 °C and 5 °C. The dry and cure times at 5 °C highlight the highly reactive nature of the product.
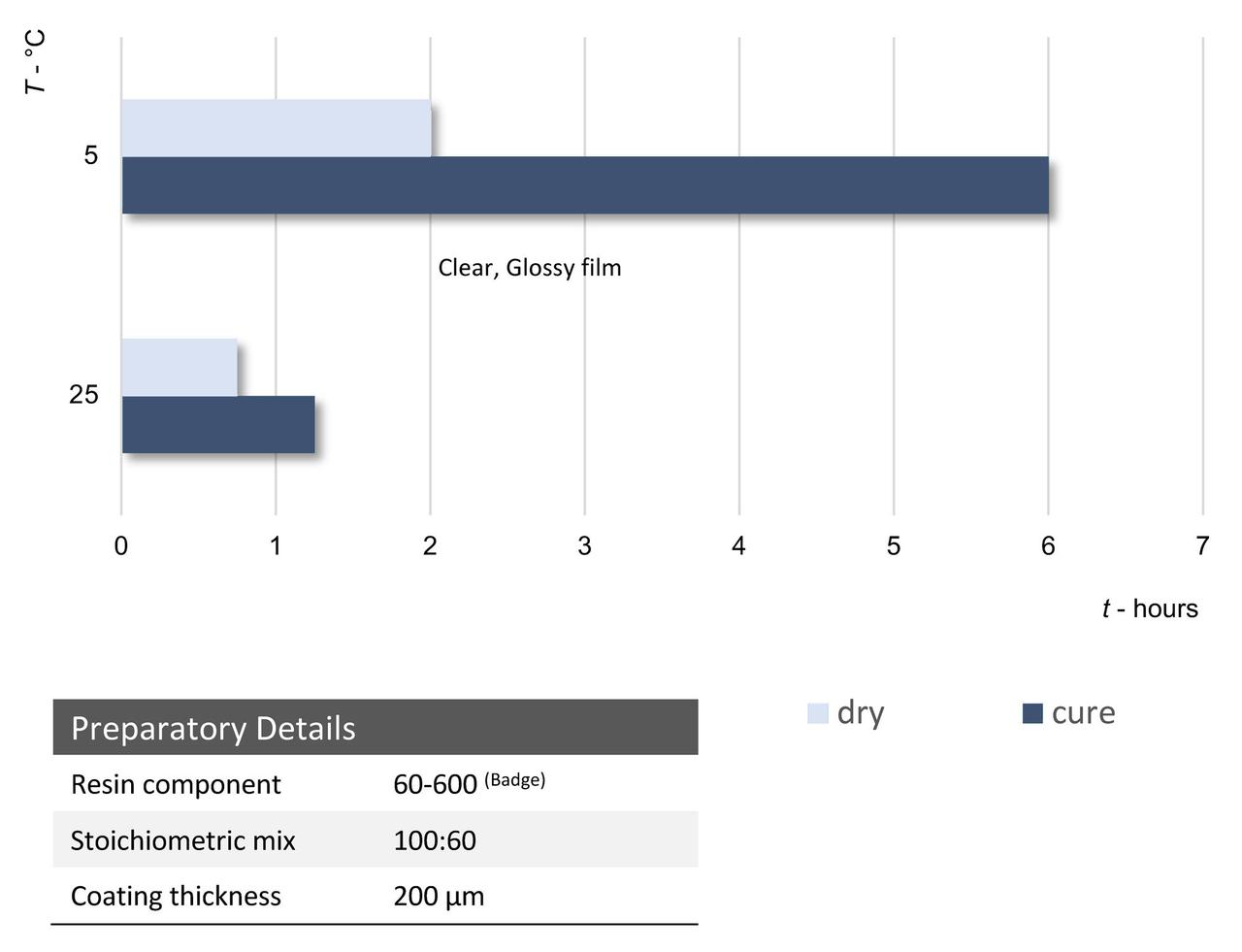
FIGURE 9 ǀ Dry times and cure times.
Summary
This innovative new series of poly-Mannich PERMACURE epoxy curing agents is formulated to offer a reduced hazard rating and variable reactivity inclusive of slow, medium and outstanding speed of cure. They are available in several forms including low-viscosity, solvent-free liquids and with high solids content. They are compliant with REACH and other regulatory requirements.
These products offer excellent reactivity profiles, and they cure under difficult, adverse conditions. They are an excellent alternative to conventional Mannich base epoxy curing agents. The latest generation of PERMACURE grades are the Mannich base of choice for future proofing your industrial system.
References
¹ Lee, H.; Neville, K. McGraw-Hill, New York, 1967.
² Jones, P, Application No. PCT/GB2020/052772, 2020.
³ European Chemicals Agency (ECHA) website https://echa.europa.eu/.
⁴ CURAMINE 31-537 TDS, technical data sheet, 2020.
⁵ CURAMINE 31-537 SDS/PERMACURE H537 SDS, 2020.
