Novel Nanocomposite Surface Additives for PFAS Replacement

–Credit: Aleksandr_Kendenkov / iStock via Getty Images Plus–
By Rich Czarnecki, Micro Powders, Tarrytown, NY
Micronized waxes were developed in the late 1960s as an advancement in surface modifying additive technology. Historically, before the innovation of micronized wax, coarser wax powders were processed through methods like ball milling to suit applications such as inks and coatings. The advent of air jet milling and controlled precipitation techniques marked the beginning of commercially available micronized waxes, which offer uniformity and enhanced performance.
Today, wax additives are an essential part of any coating formulator’s toolkit. Micronized wax powders, dispersions and emulsions can improve the durability of all types of surface coatings, improving slip and lubricity, abrasion and scratch resistance, antiblocking and rub resistance. Most commercial wax additives are based on synthetic petrochemically derived materials that include synthetic wax, polyethylene, polypropylene and polytetrafluoroethylene (PTFE). In recent years, with growing interest in coating formulations based on natural, biodegradable and sustainable feedstocks, wax powders sourced from materials such as carnauba wax, rice bran wax and even cellulose have been introduced and commercialized. The term “wax powder” has become a generic descriptor for a wide range of solid particle additives that can enhance the performance of coatings.
ADVERTISEMENT
Wax Behavior in Coatings
To understand how wax technology has progressed over the last 50 years, it is important to understand how these particles behave in paints, inks and coatings. Wax particles rise to the surface of a liquid coating (typically solvent or water based) through a combination of physical and chemical processes during application and drying or curing:
- Density: Wax particles are often less dense than the liquid medium in the coating formulation. As the coating dries or cures, heavier components settle, allowing wax particles to migrate to the top due to buoyancy effects. Additionally, waxes are typically immiscible with the resins in the coating, promoting phase separation that drives their migration to the surface.
- Film Shrinkage: As a liquid coating dries, the eventual dry film coating thickness helps to drive wax particles to the air-interface, where they will be most effective.
- Solubility and Compatibility: Low compatibility between wax (typically highly nonpolar) and the binder system (typically much more polar) contributes to the separation. As the water or solvent evaporates, the wax becomes less soluble in the shrinking liquid phase, leading to its deposition at the air-interface.
- Surface Energy Effects: Wax particles have low surface energy, which makes them naturally migrate to reduce the coating system's overall surface energy. This process enhances surface-related properties like slip, water repellency and resistance to abrasion.
This behavior is intentionally leveraged in coatings, inks and other formulations to enhance surface properties like slip, gloss control and abrasion resistance.
Wax Chemistry
The choice of wax chemistry can affect many performance properties. Table 1 provides some of the most important properties of waxes.
TABLE 1–ǀ–Important properties of waxes.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
One will note that PTFE has properties markedly different from most wax additive chemistries:
- Lowest surface energy
- Highest melting point
- Highest density
Some of these properties provide unique performance benefits in a wax additive. PTFE’s very low surface energy makes it an ideal material for imparting a high degree of slip and lubricity. The high melting point of PTFE enables use in applications where a coating undergoes a baking or oven curing process since it will not melt. Additionally, PTFE is an extremely durable material that can provide those benefits along with improvements in scratch, mar and abrasion resistance.
On the contrary, the high density of PTFE significantly impacts the ability of micronized particles to migrate efficiently to the surface of a coating after drying and curing.
Wax Composites
To overcome the high density and poor mobility of PTFE, wax manufacturers developed composite or alloy waxes that combine finely micronized PTFE with other waxes. The most popular permutation of this approach would be a wax composite based on a combination of PTFE and polyethylene wax, typically high-density polyethylene (HDPE). Such a composite wax is manufactured by blending fine PTFE powder with molten HDPE in a bulk melting or extrusion process. This intermediate composite material is then cooled, crushed and size reduced (micronized) into a fine powder. In the example below, one can see how the right combination of PTFE and HDPE can result in a multicomponent particle powder with optimized density (Table 2).
TABLE 2–ǀ–Combining materials to modify density.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
A visual depiction of a PTFE wax composite particle is shown in Figure 1, where the yellow region is the wax, and the white particles are PTFE.
FIGURE 1–ǀ–Wax/PTFE composite particle.
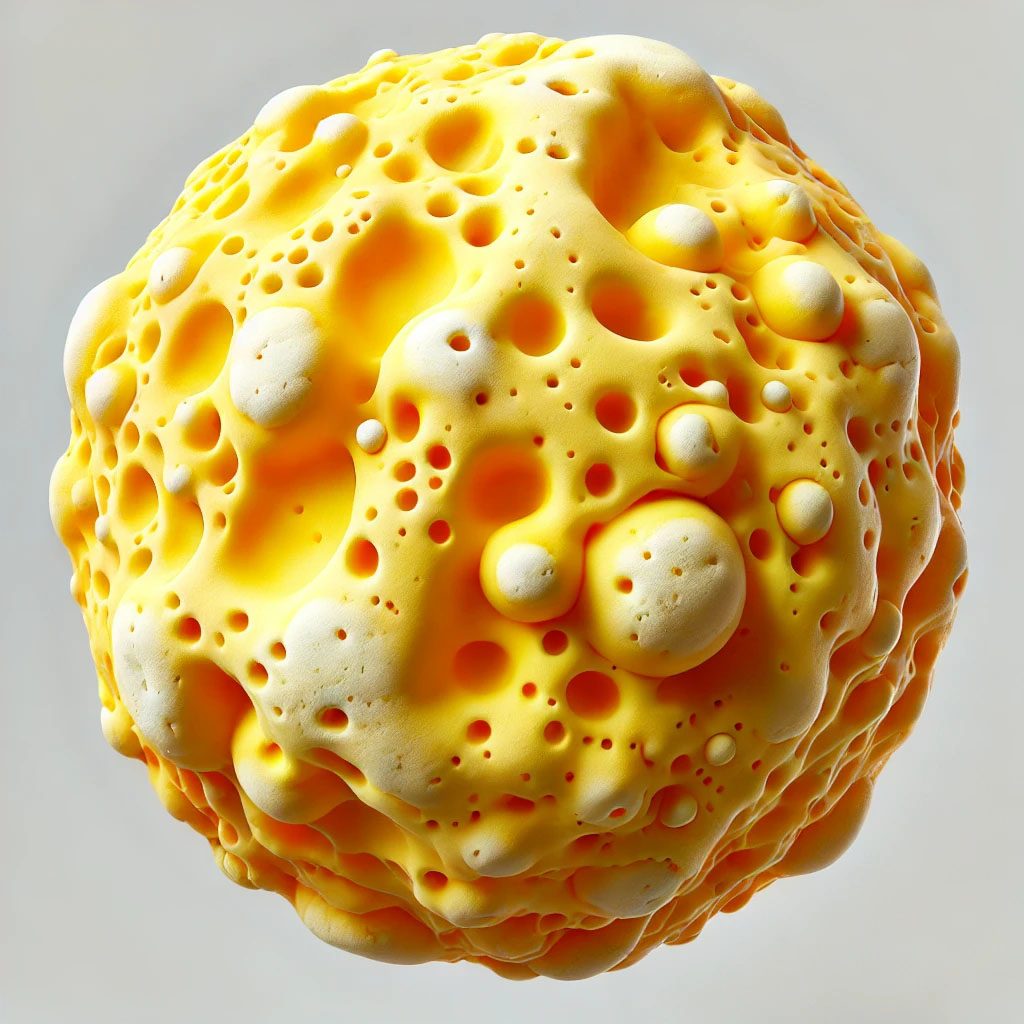
This density modification not only optimizes the mobility of the HDPE/PTFE particles, but it also improves the in-can stability of the wax particles in a water-based coating, where the density is above 1.0 (higher than water).
Wax composites based on PTFE have become one of the most important innovations in surface additive design, and many manufacturers offer high-performance products that exploit this technology. PTFE composite waxes have been established as a performance benchmark for decades in the coatings industry.
PTFE and Regulatory Activity
Per- and polyfluoroalkyl substances (PFAS) are a group of persistent organic substances that consist of a carbon chain in which hydrogen atoms are entirely or partly replaced by fluorine atoms. Perfluoroalkyl substances have a fully fluorinated carbon chain, while polyfluoroalkyl substances have a partially fluorinated carbon chain.
Since 2009, certain PFAS substances have been an increasing concern for bio-accumulative persistence in the environment. For many PFAS substances currently in use, there is a lack of detailed knowledge on their chemical structures, properties, uses and toxicological profiles. On July 15, 2021, five REACH Competent Authority Countries notified the European Chemicals Agency (ECHA) of their intention to restrict PFAS substances, including fluoropolymer substances. This legislative amendment anticipates the total ban on the use of PFAS desired by these EU Authorities due to environmental and health concerns. The proposal is targeting approximately 6,000 PFAS-related substances, which makes it the most extensive and complex restriction to date. This is a first formal step in a restriction procedure, and the future process will foresee opportunities to gather stakeholders’ input on the socio-economic impact of the restriction as well as the availability of alternatives. This proposal was submitted to ECHA on January 13, 2023. On February 7, 2023, ECHA published their PFAS restriction proposal (ECHA/NR/23/04).
On December 5, 2024, the Committees for Risk Assessment (RAC) and for Socio-Economic Analysis (SEAC) reached provisional conclusions on the proposed restriction of PFAS in three sectors:
- Construction products
- Textiles, upholstery, leather, apparel and carpets
- Food contact materials and packaging
On December 5, 2024, the Committees for Risk Assessment (RAC) and for Socio-Economic Analysis (SEAC) reached provisional conclusions on the proposed restriction of PFAS in three sectors.
Based on the tentative timeline, a new ECHA regulation on PFAS substances is not expected to enter into force (EIF) until Q3 2025 at the earliest.
In parallel, on June 28, 2021, the United States Environmental Protection Agency (EPA) published a proposed rule for broad, retroactive and prompt reporting and recordkeeping for PFAS under the Toxic Substances Control Act (TSCA). The final rule was published on October 11, 2023, and is codified as 40 CFR Part 705. The rule requires “lookback” reporting of all PFAS manufactured or imported (including in articles) in any year since Jan 1, 2011. In September 2024, EPA announced a direct final rule and a parallel proposed rule to delay the reporting period for this rule. The reporting period was scheduled to begin on November 12, 2024, but the final rule delays the beginning of the reporting period until July 2025 due to budgetary constraints.
PFAS substances can be divided into two groups: polymers and small molecules (nonpolymers). This is an important distinction due to the ecotoxicity and physicochemical behavior of nonpolymeric PFAS. Polymeric PFAS are classified as fluoropolymers, perfluorinated polyethers and side chain fluorinated polymers. Fluoropolymers’ unique physicochemical properties are distinctly different than other PFAS substances and do not display the environmental and toxicological properties associated with the other fluorochemicals. The term “fluoropolymers” refers to materials including but not limited to the denoted acronyms PTFE (polytetrafluoroethylene), FEP (fluorinated ethylene propylene), PFA (perfluoroalkyl alkanes), ETFE (ethylene tetrafluoride), ECTFE (ethylene-chlorotrifluoroethylene), PVDF (polyvinylidene fluoride), PVF (polyvinyl fluoride), fluoroelastomers, etc.
As currently written, PTFE would be included in the class described as a PFAS substance under U.S. and EU regulations. PTFE has a long history of safe use in aerospace and defense, automotive, aviation, textiles, leather and apparel, construction and household products, electronics, firefighting, food processing and medical articles. There are several PFAS sub-grouping proposals under discussion including in the U.S. by the EPA. One among them differentiates PTFE (fluoropolymers) as polymeric PFAS vs. nonpolymeric PFAS due to differences in ecotoxicity and physicochemical behavior.
The American Chemistry Council (ACC) has organized a working group under the Performance Fluoropolymer Partnership.1 PFP represents the world’s leading manufacturers, formulators or processors of fluoropolymers. PFP members promote the responsible production, use and management of fluoropolymers and advocate for a sound science- and risk-based approach to regulation.
As currently written, PTFE would be included in the class described as a PFAS substance under U.S. and EU regulations.
Industry Reaction to PTFE Regulatory Activity
While the debate continues regarding the regulation of polymeric vs. small molecule PFAS substances, many segments of the coatings industry have elected to seek alternate technologies that do not require the use of PTFE as a coatings additive. In sensitive areas like flexible and rigid food packaging, most multinationals have adopted a policy that does not allow the use of any PFAS substances, including PTFE, in new formulations. Moreover, insurance companies are becoming increasingly more concerned that PFAS could expose them to expensive claims like those seen with asbestos. They are increasingly including PFAS-specific coverage exclusions in commercial liability insurance policies.
This combination of regulatory threat and liability has led many major manufacturers of PTFE-based wax additives to announce that they will discontinue these products in the next year or so. Micro Powders will discontinue all products based on PTFE by the end of 2025.
Replacing PTFE in Composite Wax Additives
PTFE is primarily valued in composite wax powders for the following performance benefits:
- High surface lubricity (low coefficient of friction or COF)
- Excellent scratch and abrasion resistance
- Heat and chemical resistance
- Antiblocking
Fortunately, many other wax additive chemistries can also provide excellent slip and lubricity. The most important performance benefit derived by incorporating PTFE into a wax composite is generally considered to be surface durability. PTFE composite additive powders can significantly improve the resistance of a coating to damage during application, fabrication and end use. This includes scratch and abrasion resistance.
If PTFE is no longer a viable component of a wax additive, alternate materials must be identified that can bring a similar level of surface durability to a formulated coating. These materials must have a strong history of safe use, especially in food packaging.
Two approaches to replace PTFE as a component of a composite wax particle have been identified, both based on inorganic chemistry (Table 3).
TABLE 3–ǀ–Properties of inorganic materials.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
The Mohs scale is a measure of mineral hardness, ranging from 1 (talc) to 10 (diamond). Aluminum oxide (also known as alumina or corundum) measures a 9 on the Mohs scale, making it an extremely hard substance. Ceramics, although not quite as hard, are still durable materials that can be considered to replace PTFE.
Since both aluminum oxide and ceramics are hard, inert and safe materials, it was proposed that these solid particles could provide surface durability properties approaching that of PTFE in a composite wax powder. Aluminum oxide is commonly used in flooring applications as a high-durability topcoat. Floor coatings are one of the most demanding end uses, requiring maximum resistance to scratching and surface damage caused by foot traffic, furniture movement and even pets. Many high-performance floor coating formulations utilize a protective wear layer that is fortified with hard inorganic particles. Aluminum oxide has been used to impart a highly durable surface that resists wear, abrasion and scratching. With alumina at the surface of a floor coating, dramatic improvements in wear resistance can be achieved.
Aluminum oxide has been used to impart a highly durable surface that resists wear, abrasion and scratching.
The structure of fumed aluminum oxide is a complex morphology of tightly fused aggregates of nano-sized alumina particles, which subsequently attach to each other into agglomerates that are held together by weak interactions. These agglomerates can be broken down with sufficient shear energy into individual particles that can approach 300 nanometers. Since the particles are heavy, they could settle in low-viscosity coating systems, leading to potential inconsistencies in performance when applied. These particles have a very high specific surface area (SSA) and can be extremely difficult to efficiently disperse into coatings. Additionally, they are dusty, difficult to handle and could present health effects if lab or production workers are exposed to airborne dust particles.
Ceramics can include spherical materials with a mean particle size below 10 µm, as well as nanoplatelet ceramic morphologies.
Inorganic Wax Composites2
Since both aluminum oxide and ceramic particles are so dense, they will require extra energy to get them to a coating surface. As with PTFE, they will benefit from being combined with a lower density material such as HDPE in a wax composite powder. Several combinations were prepared by combining different waxes with both aluminum oxide and ceramics in an extrusion or other melt-mixing process (Table 4).
TABLE 4–ǀ–Inorganic wax composites.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
These composite materials were then air micronized to a fine particle size (Table 5).
TABLE 5–ǀ–Particle size and density of inorganic wax composites.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
You can note that the density of each wax composite has a density near or slightly higher than 1.0 to enhance efficiency and stability.
A visual depiction of an inorganic wax composite particle is shown in Figure 2, where the yellow region is the wax and the black particles are aluminum oxide.
FIGURE 2–ǀ–Wax/inorganic composite particle.
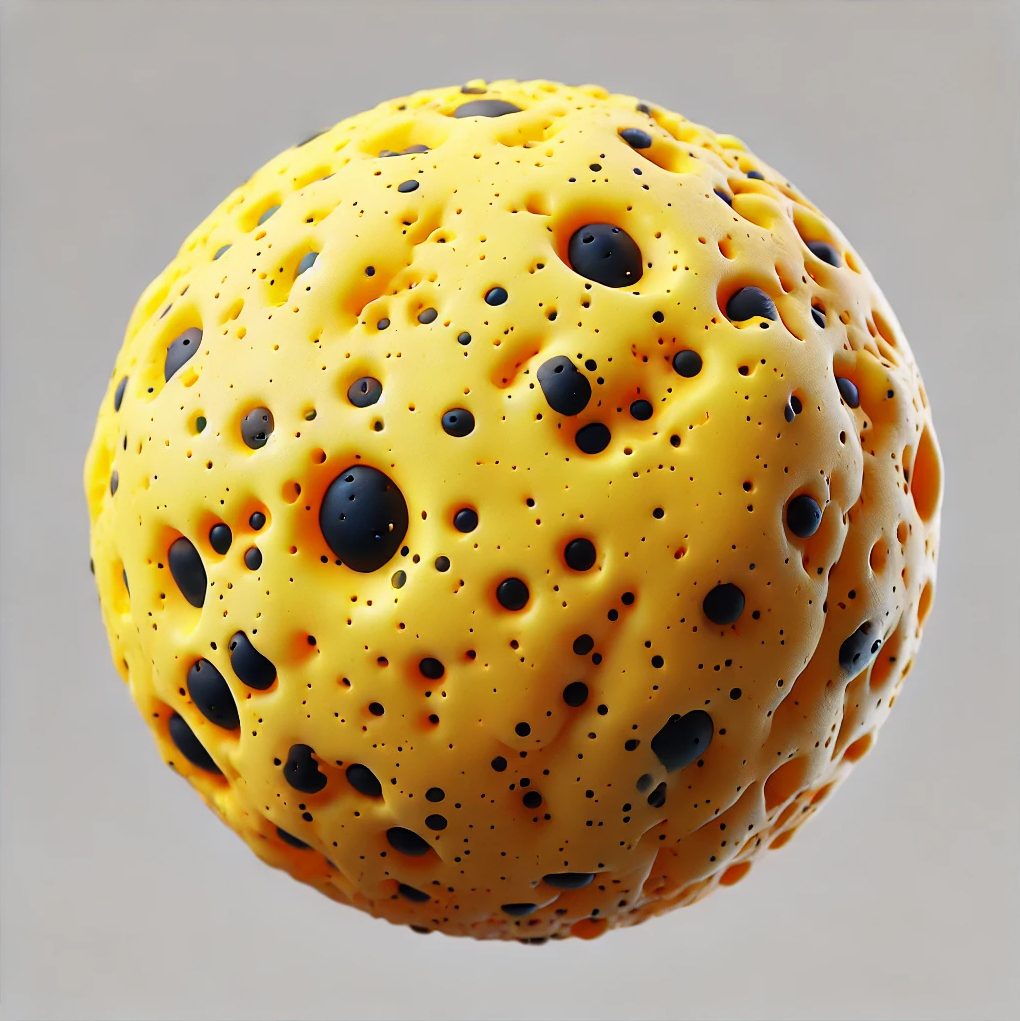
Performance — Scratch Resistance
To compare the improvement in scratch resistance, each sample was first tested against the wax counterpart without the aluminum oxide modification. All samples were dosed at 1% on total formula weight in a hard water-based polyurethane dispersion coating (Table 6) and were drawn down with a #28 wire wound rod to produce approximately 1 mil dry film thickness (DFT) coatings. Test panels were cured for 7 days at room temperature at ~50% humidity. The dried panels were tested using a Taber linear abraser (Figure 3) per ASTM D3363.
TABLE 6–ǀ–Hard water-based polyurethane dispersion coating.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
FIGURE 3–ǀ–Taber linear abraser.

ASTM D3363 uses the HB scale to measure the ability of a coating to be scratched by a pencil. The HB scale (Figure 4) uses specially formulated pencils ranging from softest (9xxB) to hardest (9H). As the pencil core becomes softer (using lower proportions of clay) it leaves a darker mark as it deposits more graphite on the paper.
FIGURE 4–ǀ–HB scale.
9H

REVERSE PINCH TO ZOOM
8H

REVERSE PINCH TO ZOOM
7H

REVERSE PINCH TO ZOOM
6H

REVERSE PINCH TO ZOOM
5H

REVERSE PINCH TO ZOOM
4H

REVERSE PINCH TO ZOOM
3H

REVERSE PINCH TO ZOOM
2H

REVERSE PINCH TO ZOOM
H

REVERSE PINCH TO ZOOM
F

REVERSE PINCH TO ZOOM
HB

REVERSE PINCH TO ZOOM
B

REVERSE PINCH TO ZOOM
2B

REVERSE PINCH TO ZOOM
3B

REVERSE PINCH TO ZOOM
4B

REVERSE PINCH TO ZOOM
5B

REVERSE PINCH TO ZOOM
6B

REVERSE PINCH TO ZOOM
7B

REVERSE PINCH TO ZOOM
8B

REVERSE PINCH TO ZOOM
9xxB

REVERSE PINCH TO ZOOM
9xxB

REVERSE PINCH TO ZOOM
Figure 5 shows the results comparing aluminum oxide modified HDPE versus the unmodified counterpart.
FIGURE 5–ǀ–ASTM D3363 pencil hardness (scratch resistance).
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
As can be seen above, the performance of the aluminum oxide-modified HDPE wax (HDPE-A) is markedly superior to the unmodified HDPE wax. Similar results showing improvement were seen with all other wax combinations, where a wax modified with either aluminum oxide or ceramic demonstrated superior performance vs. the same wax chemistry without inorganic modification.
A more relevant study is to compare the performance of aluminum oxide and ceramic modified composite waxes to classic PTFE-based composite waxes:
- HDPE/PTFE
- LDPE/PTFE
- Synthetic wax/PTFE
Pencil hardness (scratch resistance) results are shown in Figure 6.
FIGURE 6–ǀ–ASTM D3363 pencil hardness (scratch resistance).
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
The results demonstrate that wax composites based on both aluminum oxide and ceramic showed equal or better performance for scratch resistance vs. PTFE wax composites, with the ceramic-modified LDPE (LDPE-C), aluminum oxide-modified synthetic wax (SYN-A) and aluminum oxide-modified HDPE/amide wax (HDPE-AM-A) having the best results.
Performance — Abrasion Resistance
To compare the improvement in abrasion resistance, each sample was tested against PTFE composite wax powders. Each wax sample was dosed at 1% on total formula weight in a hard water-based polyurethane dispersion coating (Formula 1) and was drawn down with a #60 wire wound rod to produce approximately 2 mil (50 µm) dry film thickness (DFT) coatings. Test panels were cured for 7 days at room temperature at ~50% humidity. The dried panels were tested for abrasion resistance using a Taber rotary abrader (Figure 7) per ASTM D4060.
FIGURE 7–ǀ–Taber rotary abraser.

Figure 8 shows the results for Taber abrasion testing.
FIGURE 8–ǀ–ASTM D4060 Taber abrasion resistance after 2,500 cycles.
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
Wax composites based on both aluminum oxide and ceramic showed equal or better performance for abrasion resistance vs. PTFE wax composites, with the aluminum oxide (LDPE-A) and ceramic-modified low-density polyethylene composite (LDPE-C) having the best results. LDPE is regarded as especially good for abrasion resistance as it is more amorphous and less crystalline when compared to HDPE.
Performance of Inorganic Wax Composite vs. Free Inorganic
It was mentioned earlier that PTFE composite wax powders are more efficient (better performance) when compared to a 100% PTFE powder. To validate this for inorganic wax composites, a study was conducted where aluminum oxide is added at equal dosages to a test coating in two ways:
- As free aluminum oxide
- In a composite wax powder (LDPE-A)
Figure 9 shows the results for pencil hardness (scratch resistance).
FIGURE 9–ǀ–ASTM D3363 pencil hardness (scratch resistance).
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
X

REVERSE PINCH TO ZOOM
Keeping in mind that the test result for X% aluminum oxide (red) and 1% LDPE-A (blue) contribute the same amount of aluminum oxide to the formula, the inorganic wax composite is more effective at getting the hard aluminum oxide particles to the surface, resulting in a marked improvement in pencil scratch hardness.
Conclusion
Composite wax additives based on aluminum oxide and ceramics demonstrate excellent surface durability, often outperforming classic PTFE composite wax additives. As regulations continue to threaten the use of PFAS substances, these novel additives give formulators the ability to maintain or even improve performance while removing PTFE from their formulations.
This paper was presented at the 2025 Waterborne Symposium in New Orleans.
References
2 Czarnecki, R. J. Composite Wax Formulation Containing Polyethylene and PTFE for Coatings. U.S. Patent 10,646,212, May 12, 2020.

